Organic acid
Overview
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
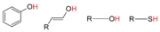
ic properties. The most common organic acids are the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s, whose acidity is associated with their carboxyl group –COOH. Sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
s, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: –OH, –SH, the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
group, and the phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
group.
Discussions

