
Nuclear reactor physics
Encyclopedia
Nuclear reactor physics is the branch of science that deals with the study and application of chain reaction to induce controlled rate of fission for energy in reactors.
Most nuclear reactors use a chain reaction
to induce a controlled rate of nuclear fission
in fissile material, releasing both energy
and free neutron
s. A reactor consists of an assembly of nuclear fuel (a reactor core
), usually surrounded by a neutron moderator
such as regular water
, heavy water
, graphite
, or zirconium hydride
, and fitted with mechanisms such as control rods that control the rate of the reaction.
The physics of nuclear fission
has several quirks that affect the design and behavior of nuclear reactors. This article presents a general overview of the physics of nuclear reactors and their behavior.
population at any instant is a function of the rate of neutron production (due to fission processes) and the rate of neutron losses (via non-fission absorption mechanisms and leakage from the system). When a reactor’s neutron population remains steady from one generation to the next (creating as many new neutrons as are lost), the fission chain reaction is self-sustaining and the reactor's condition is referred to as "critical". When the reactor’s neutron production exceeds losses, characterized by increasing power level, it is considered "supercritical", and; when losses dominate, it is considered "subcritical" and exhibits decreasing power.
The "Six-factor formula" is the neutron life-cycle balance equation, which includes six separate factors, the product of which is equal to the ratio of the number of neutrons in any generation to that of the previous one; this parameter is called the effective multiplication factor (k), a.k.a. Keff. k = LfρLthfηЄ, where Lf = "fast non-leakage factor"; ρ = "resonance escape probability"; Lth = "thermal non-leakage factor"; f = "thermal fuel utilization factor"; η = "reproduction factor"; Є = "fast-fission factor".
k = (Neutrons produced in one generation)/(Neutrons produced in the previous generation)
When the reactor is critical, k = 1. When the reactor is subcritical, k < 1. When the reactor is supercritical, k > 1.
"Reactivity
" is an expression of the departure from criticality. δk = (k - 1)/k
When the reactor is critical, δk = 0. When the reactor is subcritical, δk < 0. When the reactor is supercritical, δk > 0. Reactivity is also represented by the lowercase Greek letter rho (ρ). Reactivity is commonly expressed in decimals or percentages or pcm (per cent mille) of Δk/k. When reactivity ρ is expressed in units of delayed neutron fraction β, the unit is called the dollar.
If we write 'N' for the number of free neutrons in a reactor core and ' ' for the average lifetime of each neutron (before it either escapes from the core or is absorbed by a nucleus), then the reactor will follow differential equation
' for the average lifetime of each neutron (before it either escapes from the core or is absorbed by a nucleus), then the reactor will follow differential equation
(the evolution equation)

where is a constant of proportionality, and
is a constant of proportionality, and 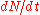 is the rate of change of the neutron count in the core. This type of differential equation describes exponential growth
is the rate of change of the neutron count in the core. This type of differential equation describes exponential growth
or exponential decay, depending on the sign of the constant , which is just the expected number of neutrons after one average neutron lifetime has elapsed:
, which is just the expected number of neutrons after one average neutron lifetime has elapsed:

Here, is the probability that a particular neutron will strike a fuel nucleus,
is the probability that a particular neutron will strike a fuel nucleus, 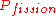 is the probability that the neutron, having struck the fuel, will cause that nucleus to undergo fission,
is the probability that the neutron, having struck the fuel, will cause that nucleus to undergo fission, 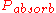 is the probability that it will be absorbed by something other than fuel, and
is the probability that it will be absorbed by something other than fuel, and  is the probability that it will "escape" by leaving the core altogether.
is the probability that it will "escape" by leaving the core altogether. 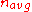 is the number of neutrons produced, on average, by a fission event—it is between 2 and 3 for both 235U and 239Pu.
is the number of neutrons produced, on average, by a fission event—it is between 2 and 3 for both 235U and 239Pu.
If is positive, then the core is supercritical and the rate of neutron production will grow exponentially until some other effect stops the growth. If
is positive, then the core is supercritical and the rate of neutron production will grow exponentially until some other effect stops the growth. If  is negative, then the core is "subcritical" and the number of free neutrons in the core will shrink exponentially until it reaches an equilibrium at zero (or the background level from spontaneous fission). If
is negative, then the core is "subcritical" and the number of free neutrons in the core will shrink exponentially until it reaches an equilibrium at zero (or the background level from spontaneous fission). If  is exactly zero, then the reactor is critical and its output does not vary in time (
is exactly zero, then the reactor is critical and its output does not vary in time (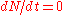 , from above).
, from above).
Nuclear reactors are engineered to reduce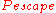 and
and  . Small, compact structures reduce the probability of direct escape by minimizing the surface area
. Small, compact structures reduce the probability of direct escape by minimizing the surface area
of the core, and some materials (such as graphite
) can reflect
some neutrons back into the core, further reducing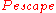 .
.
The probability of fission, , depends on the nuclear physics of the fuel, and is often expressed as a cross section
, depends on the nuclear physics of the fuel, and is often expressed as a cross section
.
Reactors are usually controlled by adjusting . Control rods made of a strongly neutron-absorbent material such as cadmium
. Control rods made of a strongly neutron-absorbent material such as cadmium
or boron
can be inserted into the core: any neutron that happens to impact the control rod is lost from the chain reaction, reducing .
. 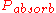 is also controlled by the recent history of the reactor core itself (see below).
is also controlled by the recent history of the reactor core itself (see below).
rate is sufficiently low it may take a long time (in 235U reactors, as long as many minutes) before a chance neutron encounter starts a chain reaction even if the reactor is supercritical. Most nuclear reactors include a "starter" neutron source
that ensures there are always a few free neutrons in the reactor core, so that a chain reaction will begin immediately when the core is made critical. A common type of startup neutron source
is a mixture of an alpha particle
emitter such as 241Am (americium-241) with a lightweight isotope such as 9Be (beryllium-9).
The primary sources described above have to be used with fresh reactor cores. For operational reactors, secondary sources are used; most often a combination of antimony
with beryllium
. Antimony becomes activated
in the reactor and produces high-energy gamma photons, which produce photoneutrons from beryllium.
Uranium-235
undergoes a small rate of natural spontaneous fission, so there are always some neutrons being produced even in a fully shutdown reactor. When the control rod
s are withdrawn and criticality is approached the number increases because the absorption of neutrons is being progressively reduced, until at criticality the chain reaction becomes self-sustaining. Note that while a neutron source is provided in the reactor, this is not essential to start the chain reaction, its main purpose is to give a shutdown neutron population which is detectable by instruments and so make the approach to critical more observable. The reactor will go critical at the same control rod position whether a source is loaded or not.
Once the chain reaction is begun, the primary starter source may be removed from the core to prevent damage from the high neutron flux
in the operating reactor core; the secondary sources usually remains in situ to provide a background reference level for control of criticality.
) will trigger an exponentially decaying chain reaction. Although the chain reaction is not self-sustaining, it acts as a multiplier that increases the equilibrium
number of neutrons in the core. This subcritical multiplication effect can be used in two ways: as a probe of how close a core is to criticality, and as a way to generate fission power without the risks associated with a critical mass.
As a measurement technique, subcritical multiplication was used during the Manhattan Project
in early experiments to determine the minimum critical masses of 235U and of 239Pu. It is still used today to calibrate the controls for nuclear reactors during startup, as many effects (discussed in the following sections) can change the required control settings to achieve criticality in a reactor. As a power-generating technique, subcritical multiplication allows generation of nuclear power for fission where a critical assembly is undesirable for safety or other reasons. A subcritical assembly together with a neutron source can serve as a steady source of heat to generate power from fission.
Including the effect of an external neutron source ("external" to the fission process, not physically external to the core), one can write a modified evolution equation:
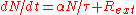
where is the rate at which the external source injects neutrons into the core. In equilibrium
is the rate at which the external source injects neutrons into the core. In equilibrium
, the core is not changing and dN/dt is zero, so the equilibrium number of neutrons is given by:
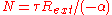
If the core is subcritical, then is negative so there is an equilibrium with a positive number of neutrons. If the core is close to criticality, then
is negative so there is an equilibrium with a positive number of neutrons. If the core is close to criticality, then  is very small and thus the final number of neutrons can be made arbitrarily large.
is very small and thus the final number of neutrons can be made arbitrarily large.
 and enable a chain reaction, uranium-fueled reactors must include a neutron moderator
and enable a chain reaction, uranium-fueled reactors must include a neutron moderator
that interacts with newly produced fast neutrons from fission events to reduce their kinetic energy from several MeV
to thermal energies of less than one eV
, making them more likely to induce fission. This is because 235U is much more likely to undergo fission when struck by one of these thermal neutrons than by a freshly produced neutron from fission.
Neutron moderators are thus materials that slow down neutrons. Neutrons are most effectively slowed by colliding with the nucleus of a light atom, hydrogen being the lightest of all. To be effective, moderator materials must thus contain light elements with atomic nuclei that tend to scatter neutrons on impact rather than absorb them. In addition to hydrogen, beryllium and carbon atoms are also suited to the job of moderating or slowing down neutrons.
Hydrogen moderators include water
(H2O), heavy water
(D
2O), and zirconium hydride
(ZrH2), all of which work because a hydrogen nucleus has nearly the same mass as a free neutron: neutron-H2O or neutron-ZrH2 impacts excite rotational modes of the molecules (spinning them around). Deuterium
nuclei (in heavy water) absorb kinetic energy less well than do light hydrogen nuclei, but they are much less likely to absorb the impacting neutron. Water or heavy water have the advantage of being transparent liquid
s, so that, in addition to shielding and moderating a reactor core, they permit direct viewing of the core in operation and can also serve as a working fluid for heat transfer.
Carbon in the form of graphite has been widely used as a moderator. It was used in Chicago Pile-1
, the world's first man-made critical assembly, and was commonplace in early reactor designs including the Soviet RBMK
nuclear power plant
s, of which the Chernobyl plant was one.
 term in the evolution equation, and more moderation reduces the effectiveness by increasing the
term in the evolution equation, and more moderation reduces the effectiveness by increasing the 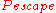 term.
term.
Most moderators become less effective with increasing temperature, so under-moderated reactors are stable against changes in temperature in the reactor core: if the core overheats, then the quality of the moderator is reduced and the reaction tends to slow down (there is a "negative temperature coefficient" in the reactivity of the core). Water is an extreme case: in extreme heat, it can boil, producing effective void
s in the reactor core without destroying the physical structure of the core; this tends to shut down the reaction and reduce the possibility of a fuel meltdown. Over-moderated reactors are unstable against changes in temperature (there is a "positive temperature coefficient" in the reactivity of the core), and so are less inherently safe than under-moderated cores.
Some reactors use a combination of moderator
materials. For example, TRIGA
type research reactors use ZrH2 moderator mixed with the 235U fuel, an H2O-filled core, and C (graphite) moderator and reflector
blocks around the periphery of the core.
. Most neutrons emitted by fission events are prompt
: they are emitted essentially instantaneously. Once emitted, the average neutron lifetime ( ) in a typical core is on the order of a millisecond
) in a typical core is on the order of a millisecond
, so if the exponential factor is as small as 0.01, then in one second the reactor power will vary by a factor of (1+0.01)1000, or more than ten thousand. Nuclear weapons are engineered to maximize the power growth rate, with lifetimes well under a millisecond and exponential factors close to 2; but such rapid variation would render it practically impossible to control the reaction rates in a nuclear reactor.
is as small as 0.01, then in one second the reactor power will vary by a factor of (1+0.01)1000, or more than ten thousand. Nuclear weapons are engineered to maximize the power growth rate, with lifetimes well under a millisecond and exponential factors close to 2; but such rapid variation would render it practically impossible to control the reaction rates in a nuclear reactor.
Fortunately, the effective neutron lifetime is much longer than the average lifetime of a single neutron in the core. About 0.65% of the neutrons produced by 235U fission, and about 0.75% of the neutrons produced by 239Pu fission, are not produced immediately, but rather are emitted by radioactive decay
of fission products, with an average lifetime of about 15 seconds. These delayed neutron
s increase the effective average lifetime of neutrons in the core, to nearly 0.1 seconds, so that a core with of 0.01 would increase in one second by only a factor of (1+0.01)10, or about 1.1 -- a 10% increase. This is a controllable rate of change.
of 0.01 would increase in one second by only a factor of (1+0.01)10, or about 1.1 -- a 10% increase. This is a controllable rate of change.
Most nuclear reactors are hence operated in a prompt subcritical, delayed critical condition: the prompt neutrons alone are not sufficient to sustain a chain reaction, but the delayed neutrons make up the small difference required to keep the reaction going. This has effects on how reactors are controlled: when a small amount of control rod is slid into or out of the reactor core, the power level changes at first very rapidly due to prompt subcritical multiplication and then more gradually, following the exponential growth or decay curve of the delayed critical reaction. Further, increases in reactor power can be performed at any desired rate simply by pulling out a sufficient length of control rod—but decreases are limited in speed, because even if the reactor is taken deeply subcritical, the delayed neutrons are produced by ordinary radioactive decay
of fission products and that decay cannot be hastened.
, because it tends to shut down (poison) an ongoing fission chain reaction. Some reactor poisons are deliberately inserted into fission reactor cores to control the reaction; boron or cadmium control rods are the best example. Many reactor poisons are produced by the fission process itself, and buildup of neutron-absorbing fission products affects both the fuel economics and the controllability of nuclear reactors.
is a useful activity: spent nuclear fuel contains about 99% of the original fissionable material present in newly manufactured nuclear fuel. Chemical separation of the fission products restores the nuclear fuel so that it can be used again.
Nuclear reprocessing is useful economically because chemical separation is much simpler to accomplish than the difficult isotope separation
required to prepare nuclear fuel from natural uranium ore, so that in principle chemical separation yields more generated energy for less effort than mining, purifying, and isotopically separating new uranium ore. In practice, both the difficulty of handling the highly radioactive fission products and other political concerns make fuel reprocessing a contentious subject. One such concern is the fact that spent uranium nuclear fuel contains significant quantities of 239Pu, a prime ingredient in nuclear weapons (see breeder reactor
).
to a stable isotope. The most important such element is xenon
, because the isotope 135Xe, a secondary fission product with a half-life of about 9 hours, is an extremely strong neutron absorber. In an operating reactor, each nucleus of 135Xe is destroyed by neutron capture
almost as soon as it is created, so that there is no buildup in the core. However, when a reactor shuts down, the level of 135Xe builds up in the core for about 9 hours before beginning to decay. The result is that, about 6–8 hours after a reactor is shut down, it can become physically impossible to restart the chain reaction until the 135Xe has had a chance to decay over the next several hours. This temporary state, which may last several days and prevent restart, is called the iodine pit
or xenon-poisoning. It is one reason why nuclear power reactors are usually operated at an even power level around the clock.
135Xe buildup in a reactor core makes it extremely dangerous to operate the reactor a few hours after it has been shut down. Because the 135Xe absorbs neutrons strongly, starting a reactor in a high-Xe condition requires pulling the control rods out of the core much farther than normal. However, if the reactor does achieve criticality, then the neutron flux in the core becomes high and 135Xe is destroyed rapidly—this has the same effect as very rapidly removing a great length of control rod from the core, and can cause the reaction to grow too rapidly or even become prompt critical
.
135Xe played a large part in the Chernobyl accident: about eight hours after a scheduled maintenance shutdown, workers tried to bring the reactor to a zero power critical
condition to test a control circuit. Since the core was loaded with 135Xe from the previous day's power generation, it was necessary to withdraw more control rods to achieve this. As a result, the overdriven reaction grew rapidly and uncontrollably, leading to steam explosion in the core, and violent destruction of the facility.
isotope found in any quantity is 235U
. About 0.7% of the uranium in most ores is the 235 isotope, and about 99.3% is the inert 238 isotope. For most uses as a nuclear fuel, uranium must be enriched - purified so that it contains a higher percentage of 235U. Because 238U absorbs fast neutrons, the critical mass
needed to sustain a chain reaction increases as the 238U content increases, reaching infinity at 94% 238U (6% 235U).
Concentrations lower than 6% 235U cannot go fast critical, though they are usable in a nuclear reactor with a neutron moderator
.
A nuclear weapon primary stage using uranium uses HEU enriched to ~90% 235U, though the secondary stage often uses lower enrichments. Nuclear reactors with water moderator can operate with only moderate enrichment of ~5% 235U. Nuclear reactors with heavy water
moderation can operate with natural uranium, eliminating altogether the need for enrichment and preventing the fuel from being useful for nuclear weapons; the CANDU
power reactors used in Canadian
power plants are an example of this type.
Uranium enrichment is difficult because the chemical properties of 235U and 238U are identical, so physical processes such as gaseous diffusion
, gas centrifuge
or mass spectrometry must be used for isotopic separation based on small differences in mass. Because enrichment is the main technical hurdle to production of nuclear fuel and simple nuclear weapons, enrichment technology is politically sensitive.
(700 million years) than 238U (4.5 billion years), so in the distant past the percentage of 235U was much higher. About two billion years ago, a water-saturated uranium deposit (in what is now the Oklo
mine in Gabon
, West Africa
) underwent a naturally occurring chain reaction that was moderated by groundwater
and, presumably, controlled by the negative void coefficient as the water boiled from the heat of the reaction. Uranium from the Oklo mine is about 50% depleted compared to other locations: it is only about 0.3% to 0.7% 235U; and the ore contains traces of stable daughters of long-decayed fission products.
Most nuclear reactors use a chain reaction
Chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events....
to induce a controlled rate of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
in fissile material, releasing both energy
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
and free neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s. A reactor consists of an assembly of nuclear fuel (a reactor core
Nuclear reactor core
A nuclear reactor core is the portion of a nuclear reactor containing the nuclear fuel components where the nuclear reactions take place.- Description :...
), usually surrounded by a neutron moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
such as regular water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
, graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, or zirconium hydride
Zirconium hydride
Zirconium hydride is an inorganic chemical compound, a hydride of zirconium with the formula ZrHx. Whereas x can be as large as 4, the most common values are between 1 and 2. Zirconium hydrides form upon reaction of zirconium metal with hydrogen gas. They behave as typical metals in terms of...
, and fitted with mechanisms such as control rods that control the rate of the reaction.
The physics of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
has several quirks that affect the design and behavior of nuclear reactors. This article presents a general overview of the physics of nuclear reactors and their behavior.
Criticality
In a nuclear reactor, the neutronNeutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
population at any instant is a function of the rate of neutron production (due to fission processes) and the rate of neutron losses (via non-fission absorption mechanisms and leakage from the system). When a reactor’s neutron population remains steady from one generation to the next (creating as many new neutrons as are lost), the fission chain reaction is self-sustaining and the reactor's condition is referred to as "critical". When the reactor’s neutron production exceeds losses, characterized by increasing power level, it is considered "supercritical", and; when losses dominate, it is considered "subcritical" and exhibits decreasing power.
The "Six-factor formula" is the neutron life-cycle balance equation, which includes six separate factors, the product of which is equal to the ratio of the number of neutrons in any generation to that of the previous one; this parameter is called the effective multiplication factor (k), a.k.a. Keff. k = LfρLthfηЄ, where Lf = "fast non-leakage factor"; ρ = "resonance escape probability"; Lth = "thermal non-leakage factor"; f = "thermal fuel utilization factor"; η = "reproduction factor"; Є = "fast-fission factor".
k = (Neutrons produced in one generation)/(Neutrons produced in the previous generation)
When the reactor is critical, k = 1. When the reactor is subcritical, k < 1. When the reactor is supercritical, k > 1.
"Reactivity
Nuclear chain reaction
A nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The specific nuclear reaction may be the fission of heavy isotopes or the fusion of light isotopes...
" is an expression of the departure from criticality. δk = (k - 1)/k
When the reactor is critical, δk = 0. When the reactor is subcritical, δk < 0. When the reactor is supercritical, δk > 0. Reactivity is also represented by the lowercase Greek letter rho (ρ). Reactivity is commonly expressed in decimals or percentages or pcm (per cent mille) of Δk/k. When reactivity ρ is expressed in units of delayed neutron fraction β, the unit is called the dollar.
If we write 'N' for the number of free neutrons in a reactor core and '
 ' for the average lifetime of each neutron (before it either escapes from the core or is absorbed by a nucleus), then the reactor will follow differential equation
' for the average lifetime of each neutron (before it either escapes from the core or is absorbed by a nucleus), then the reactor will follow differential equationDifferential equation
A differential equation is a mathematical equation for an unknown function of one or several variables that relates the values of the function itself and its derivatives of various orders...
(the evolution equation)

where
 is a constant of proportionality, and
is a constant of proportionality, and 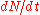 is the rate of change of the neutron count in the core. This type of differential equation describes exponential growth
is the rate of change of the neutron count in the core. This type of differential equation describes exponential growthExponential growth
Exponential growth occurs when the growth rate of a mathematical function is proportional to the function's current value...
or exponential decay, depending on the sign of the constant
 , which is just the expected number of neutrons after one average neutron lifetime has elapsed:
, which is just the expected number of neutrons after one average neutron lifetime has elapsed:
Here,
 is the probability that a particular neutron will strike a fuel nucleus,
is the probability that a particular neutron will strike a fuel nucleus, 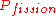 is the probability that the neutron, having struck the fuel, will cause that nucleus to undergo fission,
is the probability that the neutron, having struck the fuel, will cause that nucleus to undergo fission, 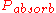 is the probability that it will be absorbed by something other than fuel, and
is the probability that it will be absorbed by something other than fuel, and  is the probability that it will "escape" by leaving the core altogether.
is the probability that it will "escape" by leaving the core altogether. 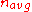 is the number of neutrons produced, on average, by a fission event—it is between 2 and 3 for both 235U and 239Pu.
is the number of neutrons produced, on average, by a fission event—it is between 2 and 3 for both 235U and 239Pu.If
 is positive, then the core is supercritical and the rate of neutron production will grow exponentially until some other effect stops the growth. If
is positive, then the core is supercritical and the rate of neutron production will grow exponentially until some other effect stops the growth. If  is negative, then the core is "subcritical" and the number of free neutrons in the core will shrink exponentially until it reaches an equilibrium at zero (or the background level from spontaneous fission). If
is negative, then the core is "subcritical" and the number of free neutrons in the core will shrink exponentially until it reaches an equilibrium at zero (or the background level from spontaneous fission). If  is exactly zero, then the reactor is critical and its output does not vary in time (
is exactly zero, then the reactor is critical and its output does not vary in time (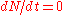 , from above).
, from above).Nuclear reactors are engineered to reduce
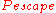 and
and  . Small, compact structures reduce the probability of direct escape by minimizing the surface area
. Small, compact structures reduce the probability of direct escape by minimizing the surface areaSurface area
Surface area is the measure of how much exposed area a solid object has, expressed in square units. Mathematical description of the surface area is considerably more involved than the definition of arc length of a curve. For polyhedra the surface area is the sum of the areas of its faces...
of the core, and some materials (such as graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
) can reflect
Reflection (physics)
Reflection is the change in direction of a wavefront at an interface between two differentmedia so that the wavefront returns into the medium from which it originated. Common examples include the reflection of light, sound and water waves...
some neutrons back into the core, further reducing
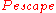 .
.The probability of fission,
 , depends on the nuclear physics of the fuel, and is often expressed as a cross section
, depends on the nuclear physics of the fuel, and is often expressed as a cross sectionCross section (physics)
A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law....
.
Reactors are usually controlled by adjusting
 . Control rods made of a strongly neutron-absorbent material such as cadmium
. Control rods made of a strongly neutron-absorbent material such as cadmiumCadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
or boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
can be inserted into the core: any neutron that happens to impact the control rod is lost from the chain reaction, reducing
 .
. 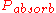 is also controlled by the recent history of the reactor core itself (see below).
is also controlled by the recent history of the reactor core itself (see below).Starter sources
The mere fact that an assembly is supercritical does not guarantee that it contains any free neutrons at all. At least one neutron is required to "strike" a chain reaction, and if the spontaneous fissionSpontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
rate is sufficiently low it may take a long time (in 235U reactors, as long as many minutes) before a chance neutron encounter starts a chain reaction even if the reactor is supercritical. Most nuclear reactors include a "starter" neutron source
Neutron source
A Neutron source is a device that emits neutrons. There is a wide variety of different sources, ranging from hand-held radioactive sources to neutron research facilities operating research reactors and spallation sources...
that ensures there are always a few free neutrons in the reactor core, so that a chain reaction will begin immediately when the core is made critical. A common type of startup neutron source
Startup neutron source
Startup neutron source is a neutron source used for stable and reliable initiation of nuclear chain reaction in nuclear reactors, when they are loaded with fresh nuclear fuel, whose neutron flux from spontaneous fission is insufficient for a reliable startup, or after prolonged shutdown periods...
is a mixture of an alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
emitter such as 241Am (americium-241) with a lightweight isotope such as 9Be (beryllium-9).
The primary sources described above have to be used with fresh reactor cores. For operational reactors, secondary sources are used; most often a combination of antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
with beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
. Antimony becomes activated
Neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha...
in the reactor and produces high-energy gamma photons, which produce photoneutrons from beryllium.
Uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
undergoes a small rate of natural spontaneous fission, so there are always some neutrons being produced even in a fully shutdown reactor. When the control rod
Control rod
A control rod is a rod made of chemical elements capable of absorbing many neutrons without fissioning themselves. They are used in nuclear reactors to control the rate of fission of uranium and plutonium...
s are withdrawn and criticality is approached the number increases because the absorption of neutrons is being progressively reduced, until at criticality the chain reaction becomes self-sustaining. Note that while a neutron source is provided in the reactor, this is not essential to start the chain reaction, its main purpose is to give a shutdown neutron population which is detectable by instruments and so make the approach to critical more observable. The reactor will go critical at the same control rod position whether a source is loaded or not.
Once the chain reaction is begun, the primary starter source may be removed from the core to prevent damage from the high neutron flux
Neutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
in the operating reactor core; the secondary sources usually remains in situ to provide a background reference level for control of criticality.
Subcritical multiplication
Even in a subcritical assembly such as a shut-down reactor core, any stray neutron that happens to be present in the core (for example from spontaneous fission of the fuel, from radioactive decay of fission products, or from a neutron sourceNeutron source
A Neutron source is a device that emits neutrons. There is a wide variety of different sources, ranging from hand-held radioactive sources to neutron research facilities operating research reactors and spallation sources...
) will trigger an exponentially decaying chain reaction. Although the chain reaction is not self-sustaining, it acts as a multiplier that increases the equilibrium
Secular equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate is equal to its decay rate.-Secular equilibrium in radioactive decay:...
number of neutrons in the core. This subcritical multiplication effect can be used in two ways: as a probe of how close a core is to criticality, and as a way to generate fission power without the risks associated with a critical mass.
As a measurement technique, subcritical multiplication was used during the Manhattan Project
Manhattan Project
The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army...
in early experiments to determine the minimum critical masses of 235U and of 239Pu. It is still used today to calibrate the controls for nuclear reactors during startup, as many effects (discussed in the following sections) can change the required control settings to achieve criticality in a reactor. As a power-generating technique, subcritical multiplication allows generation of nuclear power for fission where a critical assembly is undesirable for safety or other reasons. A subcritical assembly together with a neutron source can serve as a steady source of heat to generate power from fission.
Including the effect of an external neutron source ("external" to the fission process, not physically external to the core), one can write a modified evolution equation:
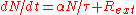
where
 is the rate at which the external source injects neutrons into the core. In equilibrium
is the rate at which the external source injects neutrons into the core. In equilibriumSecular equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate is equal to its decay rate.-Secular equilibrium in radioactive decay:...
, the core is not changing and dN/dt is zero, so the equilibrium number of neutrons is given by:
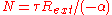
If the core is subcritical, then
 is negative so there is an equilibrium with a positive number of neutrons. If the core is close to criticality, then
is negative so there is an equilibrium with a positive number of neutrons. If the core is close to criticality, then  is very small and thus the final number of neutrons can be made arbitrarily large.
is very small and thus the final number of neutrons can be made arbitrarily large.Neutron moderators
To improve and enable a chain reaction, uranium-fueled reactors must include a neutron moderator
and enable a chain reaction, uranium-fueled reactors must include a neutron moderatorNeutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
that interacts with newly produced fast neutrons from fission events to reduce their kinetic energy from several MeV
MEV
MeV and meV are multiples and submultiples of the electron volt unit referring to 1,000,000 eV and 0.001 eV, respectively.Mev or MEV may refer to:In entertainment:* Musica Elettronica Viva, an Italian musical group...
to thermal energies of less than one eV
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
, making them more likely to induce fission. This is because 235U is much more likely to undergo fission when struck by one of these thermal neutrons than by a freshly produced neutron from fission.
Neutron moderators are thus materials that slow down neutrons. Neutrons are most effectively slowed by colliding with the nucleus of a light atom, hydrogen being the lightest of all. To be effective, moderator materials must thus contain light elements with atomic nuclei that tend to scatter neutrons on impact rather than absorb them. In addition to hydrogen, beryllium and carbon atoms are also suited to the job of moderating or slowing down neutrons.
Hydrogen moderators include water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
(H2O), heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
(D
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
2O), and zirconium hydride
Zirconium hydride
Zirconium hydride is an inorganic chemical compound, a hydride of zirconium with the formula ZrHx. Whereas x can be as large as 4, the most common values are between 1 and 2. Zirconium hydrides form upon reaction of zirconium metal with hydrogen gas. They behave as typical metals in terms of...
(ZrH2), all of which work because a hydrogen nucleus has nearly the same mass as a free neutron: neutron-H2O or neutron-ZrH2 impacts excite rotational modes of the molecules (spinning them around). Deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
nuclei (in heavy water) absorb kinetic energy less well than do light hydrogen nuclei, but they are much less likely to absorb the impacting neutron. Water or heavy water have the advantage of being transparent liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s, so that, in addition to shielding and moderating a reactor core, they permit direct viewing of the core in operation and can also serve as a working fluid for heat transfer.
Carbon in the form of graphite has been widely used as a moderator. It was used in Chicago Pile-1
Chicago Pile-1
Chicago Pile-1 was the world's first man-made nuclear reactor. CP-1 was built on a rackets court, under the abandoned west stands of the original Alonzo Stagg Field stadium, at the University of Chicago. The first self-sustaining nuclear chain reaction was initiated in CP-1 on December 2, 1942...
, the world's first man-made critical assembly, and was commonplace in early reactor designs including the Soviet RBMK
RBMK
RBMK is an initialism for the Russian reaktor bolshoy moshchnosti kanalniy which means "High Power Channel-type Reactor", and describes a class of graphite-moderated nuclear power reactor which was built in the Soviet Union. The RBMK reactor was the type involved in the Chernobyl disaster...
nuclear power plant
Nuclear power plant
A nuclear power plant is a thermal power station in which the heat source is one or more nuclear reactors. As in a conventional thermal power station the heat is used to generate steam which drives a steam turbine connected to a generator which produces electricity.Nuclear power plants are usually...
s, of which the Chernobyl plant was one.
Moderators and reactor design
The amount and nature of neutron moderation affects reactor controllability and hence safety. Because moderators both slow and absorb neutrons, there is an optimum amount of moderator to include in a given geometry of reactor core. Less moderation reduces the effectiveness by reducing the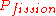 term in the evolution equation, and more moderation reduces the effectiveness by increasing the
term in the evolution equation, and more moderation reduces the effectiveness by increasing the 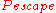 term.
term.Most moderators become less effective with increasing temperature, so under-moderated reactors are stable against changes in temperature in the reactor core: if the core overheats, then the quality of the moderator is reduced and the reaction tends to slow down (there is a "negative temperature coefficient" in the reactivity of the core). Water is an extreme case: in extreme heat, it can boil, producing effective void
Void coefficient
In nuclear engineering, the void coefficient is a number that can be used to estimate how much the reactivity of a nuclear reactor changes as voids form in the reactor moderator or coolant...
s in the reactor core without destroying the physical structure of the core; this tends to shut down the reaction and reduce the possibility of a fuel meltdown. Over-moderated reactors are unstable against changes in temperature (there is a "positive temperature coefficient" in the reactivity of the core), and so are less inherently safe than under-moderated cores.
Some reactors use a combination of moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
materials. For example, TRIGA
TRIGA
TRIGA is a class of small nuclear reactor designed and manufactured by General Atomics. The design team for TRIGA was led by the physicist Freeman Dyson.TRIGA is the acronym of Training, Research, Isotopes, General Atomics.-Design:...
type research reactors use ZrH2 moderator mixed with the 235U fuel, an H2O-filled core, and C (graphite) moderator and reflector
Neutron reflector
A neutron reflector is any material that reflects neutrons. This refers to elastic scattering rather than to a specular reflection. The material may be graphite, beryllium, steel, and tungsten carbide, or other materials...
blocks around the periphery of the core.
Delayed neutrons and controllability
Fission reactions and subsequent neutron escape happen very quickly; this is important for nuclear weapons, where the object is to make a nuclear core release as much energy as possible before it physically explodesExplosion
An explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. An explosion creates a shock wave. If the shock wave is a supersonic detonation, then the source of the blast is called a "high explosive"...
. Most neutrons emitted by fission events are prompt
Prompt neutron
In nuclear engineering, a prompt neutron is a neutron immediately emitted by a nuclear fission event, as opposed to a delayed neutron decay which can occur within the same context, emitted by one of the fission products anytime from a few milliseconds to a few minutes later.-Principle:Using U-235...
: they are emitted essentially instantaneously. Once emitted, the average neutron lifetime (
 ) in a typical core is on the order of a millisecond
) in a typical core is on the order of a millisecondMillisecond
A millisecond is a thousandth of a second.10 milliseconds are called a centisecond....
, so if the exponential factor
 is as small as 0.01, then in one second the reactor power will vary by a factor of (1+0.01)1000, or more than ten thousand. Nuclear weapons are engineered to maximize the power growth rate, with lifetimes well under a millisecond and exponential factors close to 2; but such rapid variation would render it practically impossible to control the reaction rates in a nuclear reactor.
is as small as 0.01, then in one second the reactor power will vary by a factor of (1+0.01)1000, or more than ten thousand. Nuclear weapons are engineered to maximize the power growth rate, with lifetimes well under a millisecond and exponential factors close to 2; but such rapid variation would render it practically impossible to control the reaction rates in a nuclear reactor.Fortunately, the effective neutron lifetime is much longer than the average lifetime of a single neutron in the core. About 0.65% of the neutrons produced by 235U fission, and about 0.75% of the neutrons produced by 239Pu fission, are not produced immediately, but rather are emitted by radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
of fission products, with an average lifetime of about 15 seconds. These delayed neutron
Delayed neutron
In nuclear engineering, a delayed neutron is a neutron emitted after a nuclear fission event by one of the fission products anytime from a few milliseconds to a few minutes later....
s increase the effective average lifetime of neutrons in the core, to nearly 0.1 seconds, so that a core with
 of 0.01 would increase in one second by only a factor of (1+0.01)10, or about 1.1 -- a 10% increase. This is a controllable rate of change.
of 0.01 would increase in one second by only a factor of (1+0.01)10, or about 1.1 -- a 10% increase. This is a controllable rate of change.Most nuclear reactors are hence operated in a prompt subcritical, delayed critical condition: the prompt neutrons alone are not sufficient to sustain a chain reaction, but the delayed neutrons make up the small difference required to keep the reaction going. This has effects on how reactors are controlled: when a small amount of control rod is slid into or out of the reactor core, the power level changes at first very rapidly due to prompt subcritical multiplication and then more gradually, following the exponential growth or decay curve of the delayed critical reaction. Further, increases in reactor power can be performed at any desired rate simply by pulling out a sufficient length of control rod—but decreases are limited in speed, because even if the reactor is taken deeply subcritical, the delayed neutrons are produced by ordinary radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
of fission products and that decay cannot be hastened.
Reactor poisons
Any element that strongly absorbs neutrons is called a reactor poisonNuclear poison
A neutron poison is a substance with a large neutron absorption cross-section in applications, such as nuclear reactors. In such applications, absorbing neutrons is normally an undesirable effect...
, because it tends to shut down (poison) an ongoing fission chain reaction. Some reactor poisons are deliberately inserted into fission reactor cores to control the reaction; boron or cadmium control rods are the best example. Many reactor poisons are produced by the fission process itself, and buildup of neutron-absorbing fission products affects both the fuel economics and the controllability of nuclear reactors.
Long-lived poisons and fuel reprocessing
In practice, buildup of reactor poisons in nuclear fuel is what determines the lifetime of nuclear fuel in a reactor: long before all possible fissions have taken place, buildup of long-lived neutron absorbing fission products damps out the chain reaction. This is the reason that nuclear reprocessingNuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
is a useful activity: spent nuclear fuel contains about 99% of the original fissionable material present in newly manufactured nuclear fuel. Chemical separation of the fission products restores the nuclear fuel so that it can be used again.
Nuclear reprocessing is useful economically because chemical separation is much simpler to accomplish than the difficult isotope separation
Isotope separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes, for example separating natural uranium into enriched uranium and depleted uranium. This is a crucial process in the manufacture of uranium fuel for nuclear power stations, and is...
required to prepare nuclear fuel from natural uranium ore, so that in principle chemical separation yields more generated energy for less effort than mining, purifying, and isotopically separating new uranium ore. In practice, both the difficulty of handling the highly radioactive fission products and other political concerns make fuel reprocessing a contentious subject. One such concern is the fact that spent uranium nuclear fuel contains significant quantities of 239Pu, a prime ingredient in nuclear weapons (see breeder reactor
Breeder reactor
A breeder reactor is a nuclear reactor capable of generating more fissile material than it consumes because its neutron economy is high enough to breed fissile from fertile material like uranium-238 or thorium-232. Breeders were at first considered superior because of their superior fuel economy...
).
Short-lived poisons and controllability
Short-lived reactor poisons in fission products strongly affect how nuclear reactors can operate. Unstable fission product nuclei transmute into many different elements (secondary fission products) as they undergo a decay chainDecay chain
In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...
to a stable isotope. The most important such element is xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
, because the isotope 135Xe, a secondary fission product with a half-life of about 9 hours, is an extremely strong neutron absorber. In an operating reactor, each nucleus of 135Xe is destroyed by neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
almost as soon as it is created, so that there is no buildup in the core. However, when a reactor shuts down, the level of 135Xe builds up in the core for about 9 hours before beginning to decay. The result is that, about 6–8 hours after a reactor is shut down, it can become physically impossible to restart the chain reaction until the 135Xe has had a chance to decay over the next several hours. This temporary state, which may last several days and prevent restart, is called the iodine pit
Iodine pit
Iodine pit, also called iodine hole and xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short-lived nuclear poisons in the core of a nuclear reactor. The main isotope responsible is xenon-135, mainly produced by natural decay of iodine-135. Iodine-135 is a weak neutron...
or xenon-poisoning. It is one reason why nuclear power reactors are usually operated at an even power level around the clock.
135Xe buildup in a reactor core makes it extremely dangerous to operate the reactor a few hours after it has been shut down. Because the 135Xe absorbs neutrons strongly, starting a reactor in a high-Xe condition requires pulling the control rods out of the core much farther than normal. However, if the reactor does achieve criticality, then the neutron flux in the core becomes high and 135Xe is destroyed rapidly—this has the same effect as very rapidly removing a great length of control rod from the core, and can cause the reaction to grow too rapidly or even become prompt critical
Prompt critical
In nuclear engineering, an assembly is prompt critical if for each nuclear fission event, one or more of the immediate or prompt neutrons released causes an additional fission event. This causes a rapid, exponential increase in the number of fission events...
.
135Xe played a large part in the Chernobyl accident: about eight hours after a scheduled maintenance shutdown, workers tried to bring the reactor to a zero power critical
Zero power critical
Zero power critical is a condition of nuclear fission reactors, that is useful for characterizing the reactor core. A reactor is in the zero power critical state if it is sustaining a stable fission chain reaction with no significant growth or decay in the reaction rate, and at a low enough level...
condition to test a control circuit. Since the core was loaded with 135Xe from the previous day's power generation, it was necessary to withdraw more control rods to achieve this. As a result, the overdriven reaction grew rapidly and uncontrollably, leading to steam explosion in the core, and violent destruction of the facility.
Uranium enrichment
While many fissionable isotopes exist in nature, the only usefully fissileFissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
isotope found in any quantity is 235U
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
. About 0.7% of the uranium in most ores is the 235 isotope, and about 99.3% is the inert 238 isotope. For most uses as a nuclear fuel, uranium must be enriched - purified so that it contains a higher percentage of 235U. Because 238U absorbs fast neutrons, the critical mass
Critical mass
A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The...
needed to sustain a chain reaction increases as the 238U content increases, reaching infinity at 94% 238U (6% 235U).
Concentrations lower than 6% 235U cannot go fast critical, though they are usable in a nuclear reactor with a neutron moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
.
A nuclear weapon primary stage using uranium uses HEU enriched to ~90% 235U, though the secondary stage often uses lower enrichments. Nuclear reactors with water moderator can operate with only moderate enrichment of ~5% 235U. Nuclear reactors with heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
moderation can operate with natural uranium, eliminating altogether the need for enrichment and preventing the fuel from being useful for nuclear weapons; the CANDU
CANDU reactor
The CANDU reactor is a Canadian-invented, pressurized heavy water reactor. The acronym refers to its deuterium-oxide moderator and its use of uranium fuel...
power reactors used in Canadian
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
power plants are an example of this type.
Uranium enrichment is difficult because the chemical properties of 235U and 238U are identical, so physical processes such as gaseous diffusion
Gaseous diffusion
Gaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride through semi-permeable membranes. This produces a slight separation between the molecules containing uranium-235 and uranium-238 . By use of a large cascade of many stages, high separations...
, gas centrifuge
Gas centrifuge
A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centripetal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radius of a rotating container.A prominent use of gas...
or mass spectrometry must be used for isotopic separation based on small differences in mass. Because enrichment is the main technical hurdle to production of nuclear fuel and simple nuclear weapons, enrichment technology is politically sensitive.
Oklo: a natural nuclear reactor
Modern deposits of uranium contain only up to ~0.7% 235U (and ~99.3% 238U), which is not enough to sustain a chain reaction moderated by ordinary water. But 235U has a much shorter half-lifeHalf-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
(700 million years) than 238U (4.5 billion years), so in the distant past the percentage of 235U was much higher. About two billion years ago, a water-saturated uranium deposit (in what is now the Oklo
Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African state of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.-History:...
mine in Gabon
Gabon
Gabon , officially the Gabonese Republic is a state in west central Africa sharing borders with Equatorial Guinea to the northwest, Cameroon to the north, and with the Republic of the Congo curving around the east and south. The Gulf of Guinea, an arm of the Atlantic Ocean is to the west...
, West Africa
West Africa
West Africa or Western Africa is the westernmost region of the African continent. Geopolitically, the UN definition of Western Africa includes the following 16 countries and an area of approximately 5 million square km:-Flags of West Africa:...
) underwent a naturally occurring chain reaction that was moderated by groundwater
Groundwater
Groundwater is water located beneath the ground surface in soil pore spaces and in the fractures of rock formations. A unit of rock or an unconsolidated deposit is called an aquifer when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock...
and, presumably, controlled by the negative void coefficient as the water boiled from the heat of the reaction. Uranium from the Oklo mine is about 50% depleted compared to other locations: it is only about 0.3% to 0.7% 235U; and the ore contains traces of stable daughters of long-decayed fission products.
See also
- List of nuclear reactors
- Nuclear physicsNuclear physicsNuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
- Nuclear fissionNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
- Nuclear fusionNuclear fusionNuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...

