Nitroxyl
Encyclopedia
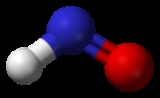
Nitroxyl is the chemical compound HNO. It is well known in the gas phase . In aqueous solution it acts as an acid with the conjugate base NO−, (pKa
= 11.4). NO− is the reduced form of nitric oxide
(NO) and is isoelectronic with dioxygen. Nitroxyl can be formed as a reaction intermediate.
Nitroxyl is very reactive towards nucleophiles (especially thiols) and quickly dimerizes to hyponitrous acid
, H2N2O2, which is then dehydrated to nitrous oxide
N2O. Therefore, HNO is generally prepared in situ
for example with the compounds such as Angeli’s salt (Na2N2O3) and Piloty’s acid (PhSO2NHOH) when it is needed.
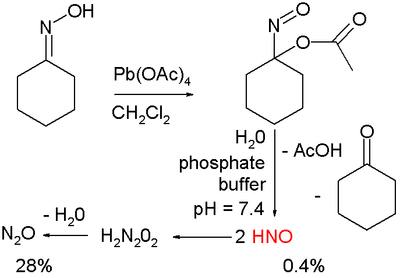
Nitroxyl shows potential in the treatment of heart failure and ongoing research is focused on finding new nitroxyl donors. In one study such donor is prepared by organic oxidation of cyclohexanone oxime
with lead tetraacetate to 1-nitrosocyclohexyl acetate:
This compound can be hydrolyzed under basic
conditions in a phosphate buffer
to nitroxyl HNO, acetic acid
and cyclohexanone
.
Other studies that have been conducted on HNO precursors include those from Nagasawa et al. in which Piloty's acid is derivatized and produces HNO upon thermal decomposition. Other notable studies on the production of HNO come from Toscano et al. in which cycloadducts of acyl nitroso species (which are known to decompose via hydrolysis to HNO and acyl acid) are synthesized. Upon photolysis these compounds release the acyl nitroso species which then further decomposes.
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
= 11.4). NO− is the reduced form of nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
(NO) and is isoelectronic with dioxygen. Nitroxyl can be formed as a reaction intermediate.
Nitroxyl is very reactive towards nucleophiles (especially thiols) and quickly dimerizes to hyponitrous acid
Hyponitrous acid
Hyponitrous acid is the chemical compound H2N2O2. This can be formulated as HON=NOH and is an isomer of nitramide, . It forms white crystals that are explosive when dry...
, H2N2O2, which is then dehydrated to nitrous oxide
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
N2O. Therefore, HNO is generally prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
for example with the compounds such as Angeli’s salt (Na2N2O3) and Piloty’s acid (PhSO2NHOH) when it is needed.
Nitroxyl shows potential in the treatment of heart failure and ongoing research is focused on finding new nitroxyl donors. In one study such donor is prepared by organic oxidation of cyclohexanone oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
with lead tetraacetate to 1-nitrosocyclohexyl acetate:
This compound can be hydrolyzed under basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
conditions in a phosphate buffer
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
to nitroxyl HNO, acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
and cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
.
Other studies that have been conducted on HNO precursors include those from Nagasawa et al. in which Piloty's acid is derivatized and produces HNO upon thermal decomposition. Other notable studies on the production of HNO come from Toscano et al. in which cycloadducts of acyl nitroso species (which are known to decompose via hydrolysis to HNO and acyl acid) are synthesized. Upon photolysis these compounds release the acyl nitroso species which then further decomposes.