
Nitroamine
Encyclopedia
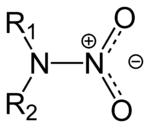
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that contain the nitroamino functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, R2N-NO2. The R's are the continuation of the ring structure, see RDX
RDX
RDX, an initialism for Research Department Explosive, is an explosive nitroamine widely used in military and industrial applications. It was developed as an explosive which was more powerful than TNT, and it saw wide use in WWII. RDX is also known as cyclonite, hexogen , and T4...
. Examples of nitroamines are the explosives HMX
HMX
HMX, also called octogen, is a powerful and relatively insensitive nitroamine high explosive, chemically related to RDX. Like RDX, the name has been variously listed as High Melting eXplosive, Her Majesty's eXplosive, High-velocity Military eXplosive, or High-Molecular-weight rdX.The molecular...
, cyclotrimethylene trinitramine
RDX
RDX, an initialism for Research Department Explosive, is an explosive nitroamine widely used in military and industrial applications. It was developed as an explosive which was more powerful than TNT, and it saw wide use in WWII. RDX is also known as cyclonite, hexogen , and T4...
, Hexanitrohexaazaisowurtzitane
Hexanitrohexaazaisowurtzitane
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane, also called HNIW, and CL-20, is a nitroamine explosive with the formula C6H6N12O12, developed by the China Lake facility, primarily to be used in propellants. It has a better oxidizer-to-fuel ratio than conventional HMX or RDX...
and tetryl
Tetryl
2,4,6-Trinitrophenylmethylnitramine commonly referred to as tetryl is a sensitive explosive compound used to make detonators and explosive booster charges....
. Nitroamines are not to be confused with nitrosamine
Nitrosamine
Nitrosamines are chemical compounds of the chemical structure R1N-N=O, some of which are carcinogenic.-Usages:Nitrosamines are used in manufacture of some cosmetics, pesticides, and in most rubber products. -Occurrences:...
s (with an 's').
Despite their amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group they are not basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
due to the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
withdrawing effect of the nitro functional group. Secondary nitroamines of the structure R-NH-NO2 are actually weak organic acid
Organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate...
s with a pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of around 5.6.

