Nitrene
Encyclopedia
In chemistry
, a nitrene (R-N:) is the nitrogen
analogue of a carbene
. The nitrogen atom
has only 6 valence electron
s and is therefore considered an electrophile
. A nitrene is a reactive intermediate
and is involved is many chemical reaction
s.
with hydrogen
, two other create a free electron pair and the two remaining electrons occupy two degenerate p orbitals. Consistent with Hund's rule the low energy form of imidogene is a triplet
with one electron in each of the p orbitals and the high energy form is the singlet state with an electron pair filling one p orbital and the other one vacant.
As with carbenes, a strong correlation exists between the spin density on the nitrogen atom which can be calculated in silico
and the zero-field splitting parameter D which can be derived experimentally from electron spin resonance. Small nitrenes such as NH or CF3N have D values around 1.8 cm−1 with spin densities close to a maximum value of 2. At the lower end of the scale are molecules with low D (< 0.4) values and spin density of 1.2 to 1-4 such as 9-anthrylnitrene and 9-phenanthryl.
In most cases, however, [N-(p-nitrophenylsulfonyl)imino]phenyliodinane (Ph=INNs) is prepared separately as follows:
Nitrene transfer takes place next:
radical group incorporated, another system has a carbon radical group.
In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a sigma, sigma, pi-triradical. A carbene
nitrogen radical (imidyl radical) resonance structure makes a contribution to the total electronic picture.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a nitrene (R-N:) is the nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
analogue of a carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
. The nitrogen atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
has only 6 valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s and is therefore considered an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
. A nitrene is a reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
and is involved is many chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s.
Electron configuration
In the most simple nitrene linear imidogene (:N-H) two of the 6 available electrons form a covalent bondCovalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
with hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, two other create a free electron pair and the two remaining electrons occupy two degenerate p orbitals. Consistent with Hund's rule the low energy form of imidogene is a triplet
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
with one electron in each of the p orbitals and the high energy form is the singlet state with an electron pair filling one p orbital and the other one vacant.
As with carbenes, a strong correlation exists between the spin density on the nitrogen atom which can be calculated in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
and the zero-field splitting parameter D which can be derived experimentally from electron spin resonance. Small nitrenes such as NH or CF3N have D values around 1.8 cm−1 with spin densities close to a maximum value of 2. At the lower end of the scale are molecules with low D (< 0.4) values and spin density of 1.2 to 1-4 such as 9-anthrylnitrene and 9-phenanthryl.
Formation
Because nitrenes are so reactive, they are not isolated. Instead, they are formed as reactive intermediates during a reaction. There are two common ways to generate nitrenes:- from azideAzideAzide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
s by thermolysis or photolysis, with expulsion of nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
gas. This method is analogous to the formation of carbeneCarbeneIn chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s from diazo compounds. - from isocyanateIsocyanateIsocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
s, with expulsion of carbon monoxideCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. This method is analogous to the formation of carbenes from keteneKeteneA ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
s.
Reactions
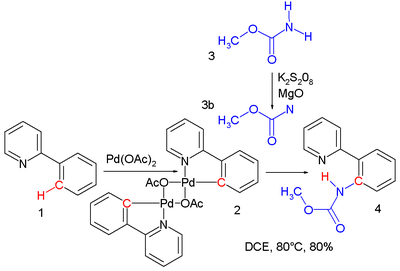
Nitrene reactions include:- nitrene C-H insertion. A nitrene can easily insert into a carbon to hydrogen covalent bondCovalent bondA covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
yielding an amine or amide. A singlet nitrene reacts with retention of configuration. In one study a nitrene, formed by oxidation of a carbamateCarbamateCarbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
with potassium persulfate, gives an insertion reactionInsertion reactionAn insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:...
into the palladiumPalladiumPalladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
to nitrogen bond of the reaction product of palladium(II) acetatePalladium(II) acetatePalladium acetate is a chemical compound of palladium described by the formula Pd2 or Pd2. It is considered more reactive than the analogous platinum compound...
with 2-phenylpyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to methyl N-(2-pyridylphenyl)carbamate in a cascade reactionCascade reactionA cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
:
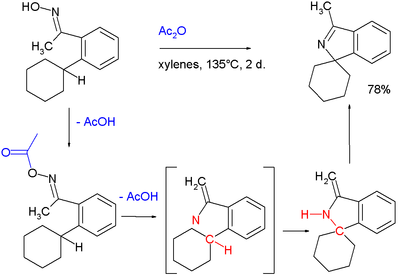
- A nitrene intermediate is suspected in this C-H insertion involving an oximeOximeAn oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
, acetic anhydrideAcetic anhydrideAcetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
leading to an isoindoleIsoindoleIsoindole in heterocyclic chemistry is a benzo fused pyrrole. The compound is an isomer of indole and its reduced form is an isoindoline.In solution its tautomer without full aromaticity over the whole ring system is predominant:...
:
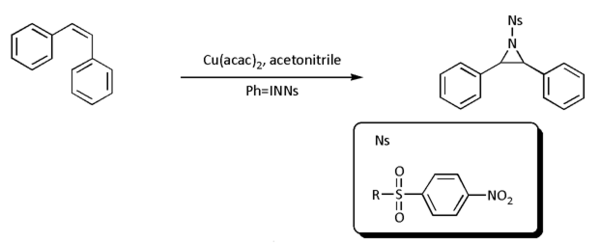
- nitrene cycloaddition. With alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s, nitrenes react to aziridineAziridineAziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
s, very often with nitrenoid precursors such as nosyl- or tosyl-substituted [N-(phenylsulfonyl)imino]phenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the sulfonamideSulfonamide (chemistry)In chemistry, the sulfonamide functional group is -S2-NH2, a sulfonyl group connected to an amine group.A sulfonamide is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2...
in presence of a transition metalTransition metalThe term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
based catalyst such as copperCopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, palladiumPalladiumPalladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
, or goldGoldGold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
:
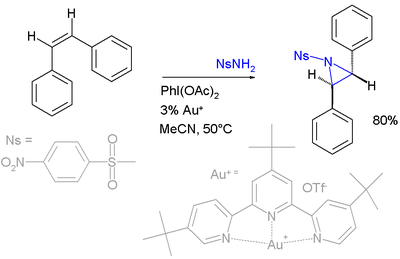
In most cases, however, [N-(p-nitrophenylsulfonyl)imino]phenyliodinane (Ph=INNs) is prepared separately as follows:
Nitrene transfer takes place next:
- In this particular reaction both the cis stilbeneStilbene-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
illustrated and the trans form (not depicted) result in the same trans-aziridine product, suggesting a two-step reaction mechanismReaction mechanismIn chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
. The energy difference between triplet and singlet nitrenes can be very small in some cases, allowing interconversionIntersystem crossingIntersystem crossing is a radiationless process involving a transition between two electronic states with different spin multiplicity.-Singlet and triplet states:...
at room temperature. Triplet nitrenes are thermodynamically more stable but react stepwise allowing free rotation and thus producing a mixture of stereochemistry.- arylnitrene ring-expansion and ring-contraction. ArylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
nitrenes show ring expansion to 7-membered ring cumuleneCumuleneA cumulene is a chemical compound with two or more cumulative double bonds, for example butatriene , H2C=C=C=CH2. Unlike alkanes and most alkenes, cumulenes tend to be rigid, which makes them appealing for molecular nanotechnology. Polyynes are another kind of rigid carbon chains...
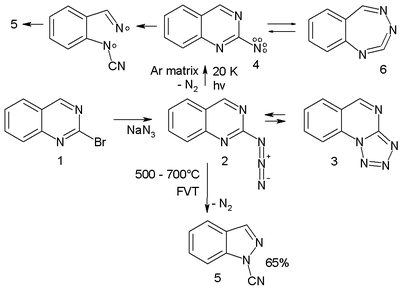
s, ring opening reactions and nitrile formations many times in complex reaction paths. For instance the azideAzideAzide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
2 in the scheme below trapped in an argonArgonArgon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
matrixMatrix IsolationMatrix isolation is an experimental technique used in chemistry and physics which generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles are embedded. The guest is said to be isolated within the host matrix...
at 20 K on photolysis expels nitrogen to the triplet nitrene 4 (observed experimentally with ESR and ultraviolet-visible spectroscopyUltraviolet-visible spectroscopyUltraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges...
) which is in equilibrium with the ring-expansion product 6.
- arylnitrene ring-expansion and ring-contraction. Aryl
- The nitrene ultimately converts to the ring-opened nitrileNitrileA nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
5 through the diradicalDiradicalA diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
intermediate 7. In a high-temperature reaction, FVT at 500–600 °C also yields the nitrile 5 in 65% yield.
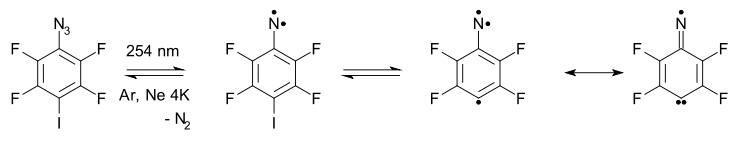
Nitreno radicals
For several compounds containing both a nitrene group and a free radical group a ESR high-spin quartet has been recorded (matrix, crygenic temperatures). One of these has a amine oxideAmine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
radical group incorporated, another system has a carbon radical group.
In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a sigma, sigma, pi-triradical. A carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
nitrogen radical (imidyl radical) resonance structure makes a contribution to the total electronic picture.