
Nicolaou Taxol total synthesis
Encyclopedia
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of Taxol. This organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
was included in Nicolaou's book, 'Classics in Total Synthesis'.
Taxol is an important drug
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
in the treatment of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.
This synthetic route to Taxol is one of several; other groups have presented their own solutions, notably the group of Holton
Holton Taxol total synthesis
The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
with a linear synthesis starting from borneol
Borneol
Borneol is a bicyclic organic compound and a terpene. The hydroxyl group in this compound is placed in an endo position.Borneol is easily oxidized to the ketone yielding camphor. One historical name for borneol is Borneo camphor which explains the name. Borneol can be synthesized by reduction of...
, the Samuel Danishefsky group starting from the Wieland-Miescher ketone
Wieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
and the Wender
Wender Taxol total synthesis
The Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul A. Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally...
group from pinene
Pinene
Pinene is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous...
.
The Nicolaou synthesis is a good example of convergent synthesis
Convergent synthesis
In chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis...
because the molecule is assembled from 3 pre-assembled synthons. Two major parts are cyclohexene rings A and C that are connected by two short bridges creating an 8 membered ring in the middle (ring B). The third pre-assembled part is an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
tail. Ring
D is an oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
ring fused to ring C. Two key chemical transformations are the Shapiro reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
and the pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
.
The overall synthesis was published in 1995 in a series of four papers in the Journal of the American Chemical Society
Journal of the American Chemical Society
The Journal of the American Chemical Society is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the Journal of Analytical and Applied Chemistry and the American Chemical Journal...
.
Retrosynthesis
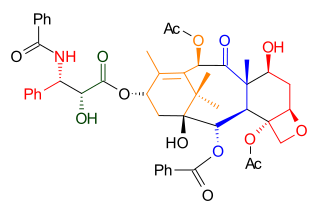
As illustrated in Retrosynthetic Scheme I, Taxol was derived from diol 7.2 by an ester bond formation, according to the Ojima-Holton method. This diol comes from carbonate 6.3 by the addition of phenyllithiumPhenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
. The oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
ring in compound 6.3 was obtained via an SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
involving a mesylate
Mesylate
In chemistry, a mesylate is any salt or ester of methanesulfonic acid . In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate .Mesylate esters are a...
derived from acetate 4.9. Ring B was closed via a McMurry reaction
McMurry reaction
The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to an alkene using titanium chloride compound such as titanium chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the...
involving dialdehyde 4.8 which ultimately was derived from aldehyde 4.2 and hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
3.6 using a Shapiro coupling reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
.
 |
| Retrosynthesis Scheme 1 |
|---|
Retrosynthetic Scheme II indicates that both the aldehyde and the hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
used in the Shapiro coupling reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
were synthesized using Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s.
 |
| Retrosynthesis Scheme 2 |
|---|
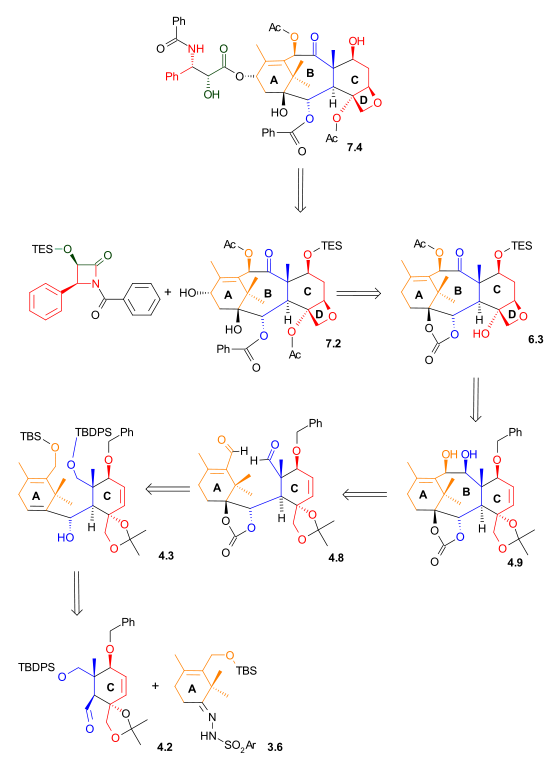
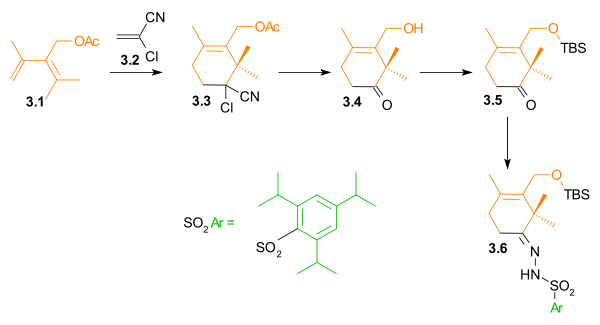
C Ring synthesis
As shown in Scheme 1, the ring synthesis of ring C began with a Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
between diene 1.3 and dienophile 1.1 in the presence of phenylboronic acid (1.2), which, after addition of 2,2-dimethyl-1,3-propanediol, gave five-membered lactone 1.8 in 62% yield. Boron served as a molecular tether and aligned both diene and dienophile for this endo Diels-Alder cycloaddition. After protection of the hydroxyl groups as tert-butyldimethylsilyl ethers, reduction of the ester with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
and selective deprotection of the secondary hydroxyl group gave lactone diol 1.11. The unusual lactone hydrates 1.9 and 1.10 were isolated as synthetic intermediates in this process.
 |
| Scheme 1 |
|---|
Lactone diol 2.1, after selective protection, was reduced with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
to give triol 2.4. This triol, after protection as the 5-membered ring acetonide, was selectively oxidized to the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
using tetrapropylammonium perruthenate (TPAP) and N-methylmorpholine N-oxide
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
. Aldehyde 2.6 served as a starting point for the construction of ring B (Scheme 4, compound 4.2).
 |
| Scheme 2 |
|---|
A Ring synthesis
The A ring synthesis (Scheme 3) started with a Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
of diene 3.1 with the commercially available dienophile 2-chloroacrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
3.2 to give cyclohexene 3.3 with complete regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
. Hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the cyanochloro group and simultaneous cleavage of the acetate group led to hydroxyketone 3.4. The hydroxyl group was protected as a tert-butyldimethylsilyl ether
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
(3.5). In preparation for a Shapiro reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
, this ketone was converted to hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
3.6.
 |
| Scheme 3 |
|---|
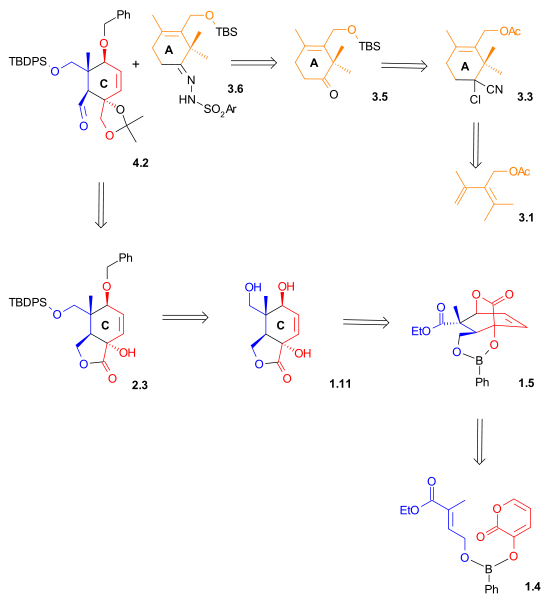
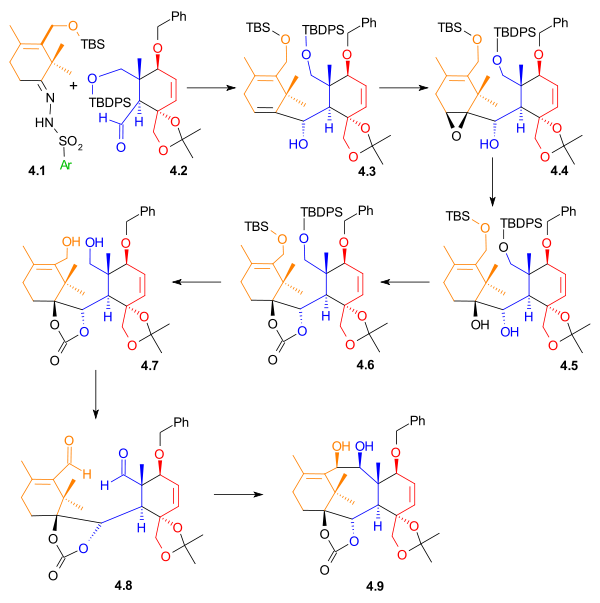
B Ring synthesis
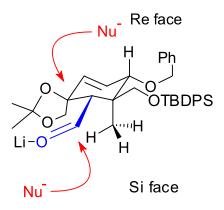
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of a vinyllithium compound to an aldehyde and the other connection through a pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
of two aldehydes (Scheme 4).
A Shapiro reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
of the vinyllithium compound derived from hydrazone 4.1 with aldehyde 4.2 makes the first connection that will become the B ring. The control of stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
in 4.3 is thought to be derived from the relative hindrance of the Si face in the orientation shown on the right, due to the proximity of the axial methyl group. Epoxidation with vanadyl(acetylacetate) converted alkene 4.5 to epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
4.4, which, upon reduction with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, gave diol 4.5. This diol was then protected as carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
4.6. The carbonate group also served to create rigidity in the ring structure for the imminent pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
. The two silyl ether
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
groups were removed, and diol 4.7 was then oxidized to give dialdehyde 4.8 using N-methylmorpholine N-oxide
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
in the presence of a catalytic amount of tetrapropylammonium perruthenate. In the final step of the formation of Ring B, a pinacol coupling
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
using conditions developed by McMurry
McMurry reaction
The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to an alkene using titanium chloride compound such as titanium chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the...
(titanium(III) chloride
Titanium(III) chloride
Titanium chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known...
and a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
/copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
) gave diol 4.9.
 |
| Scheme 4 |
|---|
Resolution
At this point in the synthesis of Taxol, the material was a racemic mixture. To obtain the desired enantiomerEnantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
, allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
4.9 was acylated
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
with (1S)-(-)-camphanic chloride and dimethylaminopyridine, giving two diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s. These were then separated using standard column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
. The desired enantiomer was then isolated when one of the separated disatereomers was treated with potassium bicarbonate
Potassium bicarbonate
Potassium bicarbonate , is a colorless, odorless, slightly basic, salty substance...
in methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
.
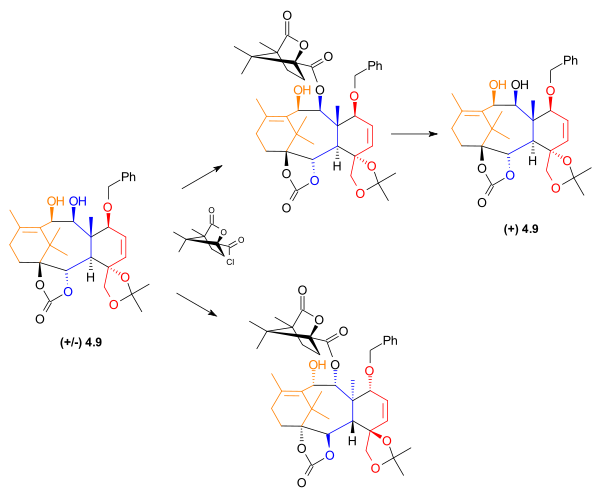 |
| Enantiomeric resolution of 4.9. |
D Ring synthesis
The desired enantiomer from resolution, allylAllyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
5.1 (Scheme 5) was acetylated
Acetylation
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound...
with acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
and 4-(dimethylamino)pyridine in methylene chloride to yield monoacetate 5.2. It is noteworthy that this reaction was exclusive for the allylic alcohol, and the adjacent hydroxyl group was not acetylated
Acetylation
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound...
. Alcohol 5.2 was oxidized
Alcohol oxidation
Alcohol oxidation is an important organic reaction. Primary alcohols can be oxidized either to aldehydes or to carboxylic acids , while the oxidation of secondary alcohols normally terminates at the ketone stage...
with tetrapropylammonium perruthenate and N-methylmorpholine N-oxide
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
to give ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
5.3. Alkene 5.3 underwent hydroboration
Hydroboration-oxidation reaction
In organic chemistry, the hydroboration–oxidation reaction is a two-step organic reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to cis stereochemistry...
in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
. Oxidation with basic hydrogen peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
and sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
gave alcohol 5.4 in 35% yield, with 15% yield of a regioisomer. The acetonide was removed, giving triol
Triol
A triol is a chemical compound containing three hydroxyl groups , such as glycerol. An example of triol on benzene is benzenetriol.- See also :* Alcohols, chemical compounds with one hydroxyl group...
5.5. This alcohol was monoacetylated, to give acetate 5.6. The benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
group was removed and replaced with a triethylsilyl group. Diol 5.7 was selectively activated using methanesulfonyl chloride
Methanesulfonyl chloride
Methanesulfonyl chloride is a compound containing a sulfonyl chloride used to make methanesulfonates and to generate sulfene.-Preparation, manufacture and handling:Methanesulfonyl chloride is highly toxic, moisture sensitive, corrosive, and a lachrymator...
and 4-(dimethylamino)pyridine to give mesylate
Mesylate
In chemistry, a mesylate is any salt or ester of methanesulfonic acid . In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate .Mesylate esters are a...
5.8, in 78% yield.
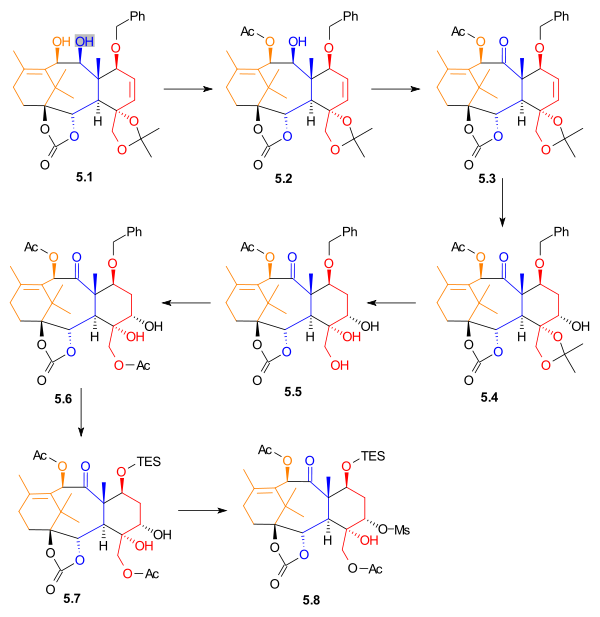 |
| Scheme 5 |
|---|
The acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
group in 6.1 (Scheme 6) was removed to give primary alcohol 6.2. The Taxol ring (D) was added by an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
nucleophilic substitution involving this hydroxyl group to give oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
6.3. After acetylation, phenyllithium
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
was used to open the carbonate ester ring to give alcohol 6.5. Allylic oxidation with pyridinium chlorochromate
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
, sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
, and celite gave ketone 6.6, which was subsequently reduced using sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
to give secondary alcohol 6.7. This was the last compound before the addition of the amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
tail.
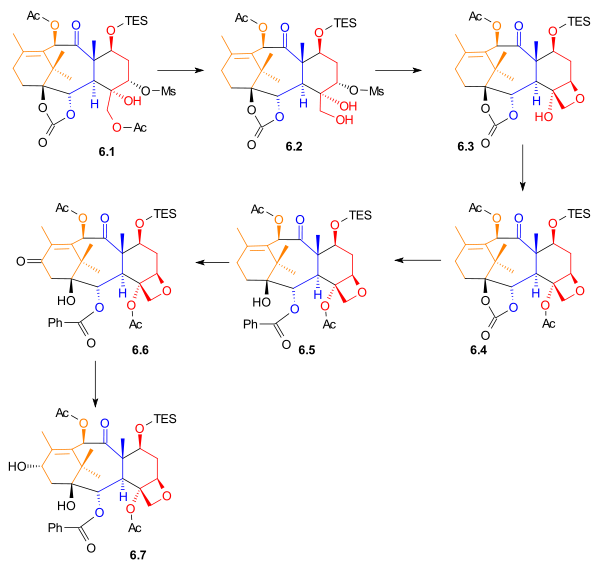 |
| Scheme 6 |
|---|
Tail addition
As shown in Scheme 7, Ojima lactamOjima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
7.1 reacted with alcohol 7.2 with sodium bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide
Sodium bisamide is the chemical compound with the formula 2NNa. This species, usually called NaHMDS , is a strong base used for deprotonation reactions or base catalyzed reaction...
as a base. This alcohol is the triethylsilyl ether of the naturally occurring compound baccatin III. The related compound, 10-deacetylbaccatin III, is found in Taxus baccata
Taxus baccata
Taxus baccata is a conifer native to western, central and southern Europe, northwest Africa, northern Iran and southwest Asia. It is the tree originally known as yew, though with other related trees becoming known, it may be now known as the English yew, or European yew.-Description:It is a small-...
, also known as the European Yew, in concentrations of 1 gram
Gram
The gram is a metric system unit of mass....
per kilogram
Kilogram
The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water...
leaves. Removal of the triethylsilyl protecting group gave Taxol.
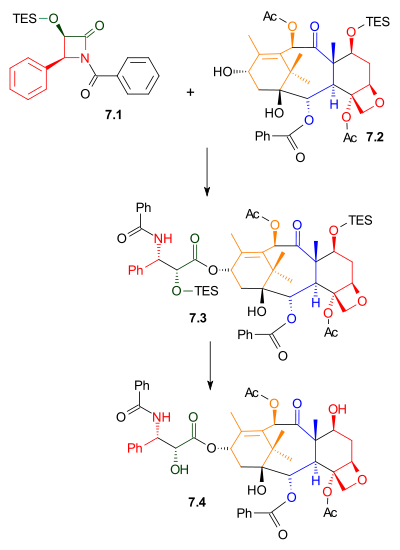 |
| Scheme 7 |
|---|
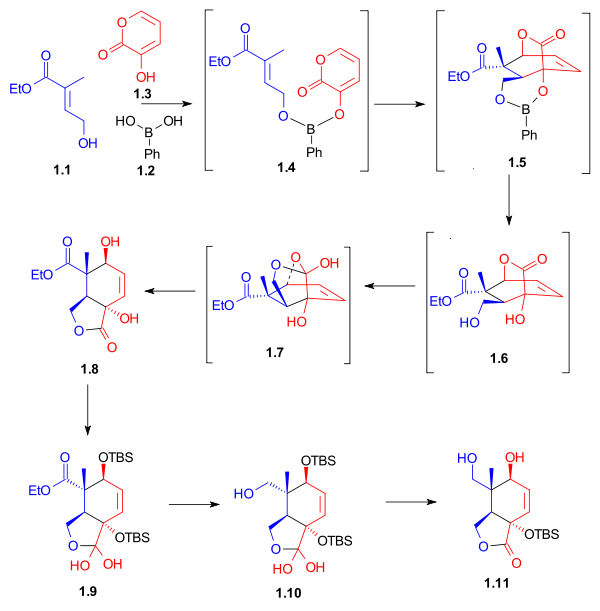
Synthesis of the Diels-Alder dienophile for Ring C
The ethyl esterEster
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
of propionic acid
Propionic acid
Propanoic acid is a naturally occurring carboxylic acid with chemical formula CH3CH2COOH. It is a clear liquid with a pungent odor...
(1) was brominated and then converted to the Wittig reagent using triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
. Aldehyde 6 was obtained from allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
(4) by protection as the tert-butyldiphenylsilyl ether
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
(5) followed by ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
. Wittig reagent 3 and aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
6 reacted in a Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
to give unsaturated ester 7, which was deprotected to give dienophile 8 (Scheme 1, compound 1).
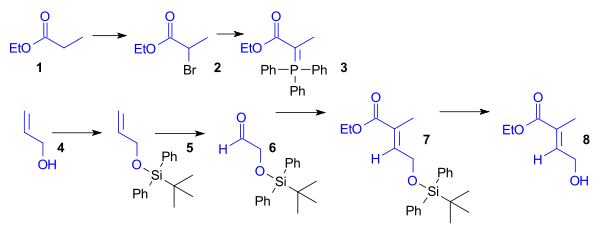
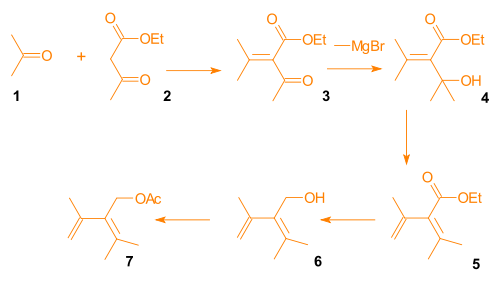
Synthesis of the Diels-Alder diene for Ring A
Aldol condensationAldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
of acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
and ethyl acetoacetate
Ethyl acetoacetate
The organic compound ethyl acetoacetate is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B1; as well as...
gave β-keto-ester 3. A Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
involving methylmagnesium bromide provided alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
4, which was subjected to acid catalyzed elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to give diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
5. Reduction and acylation
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
gave diene 7 (Scheme 3, compound 1).
Ac (acetyl)
Protection: Acetic anhydrideAcetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, 4-(dimethylamino)pyridine, and dicholoromethane
Deprotection: Potassium carbonate in methanol and water solvent
Protection prevented mesylation of the primary oxygen in 5.8.
Acetonide
Protection: 2,2-dimethoxypropane2,2-Dimethoxypropane
2,2-Dimethoxypropane or acetone dimethyl acetal or DMP is an organic compound and an alkylating reagent. The chemical formula is C5H12O2 and the molecular formula is 2C2. It is the acetalisation product of acetone and methanol. Dimethoxypropane is an intermediate for the synthesis of...
and camphorsulfonic acid
Camphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is a organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that exists as a colourless solid that is soluble in organic solvents....
, and dichloromethane
Deprotection: Hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, methanol, water, and ethyl ether
Protection of the vicinal diol 2.4 allowed the remaining hydroxyl group in alcohol 2.5 to be selectively oxidized to give aldehyde 2.6. The acetonide was removed much later in the synthesis in preparation for the closure of ring D.
Bn (benzyl)
Protection: Potassium hydridePotassium hydride
Potassium hydride, KH, is a chemical compound of potassium and hydrogen. It is a hydride of potassium. It reacts with water according to the reaction:...
, tetra-n-butylammonium iodide, and benzyl bromide
Benzyl bromide
Benzyl bromide, or α-bromotoluene, is an organic compound consisting of a benzene ring substituted with a bromomethyl group. It can be prepared by the bromination of toluene at room temperature in air, using manganese oxide as a heterogeneous catalyst...
.
Deprotection: Hydrogen, Pd(OH)2/C
Secondary alcohol 2.2 was protected as the benzyl ether so that the reduction of lactone 2.3 could occur. Protection was removed much later in the synthesis to form alcohol 5.7, which was reprotected as the triethylsilyl ether.
Carbonate Ester
Protection: Potassium hydridePotassium hydride
Potassium hydride, KH, is a chemical compound of potassium and hydrogen. It is a hydride of potassium. It reacts with water according to the reaction:...
, phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
Deprotection: Phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
opens the carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
ring to give alcohol 6.5.
Protection adds rigidity in ring structure for pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
forming diol 4.9, and also prevents unwanted oxidation in the formation of dialdehyde 4.8.
TBDPS (t-butyldiphenylsilyl)
Protection: Tert-butyldiphenylsilyl chloride, imidazoleImidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
, and dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
.
Deprotection: Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride or TBAF is a quaternary ammonium salt with the chemical formula 4N+F-. It is commercially available as the trihydrate and as a solution in tetrahydrofuran....
Primary alcohol 2.1 was protected in preparation for lactone reduction in 2.3. The protecting group was removed to give diol 4.7 in preparation for the pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
.
TBS (tert-butyldimethylsilyl) [1]
Protection: Tert-butyldimethylsilyl triflate, lutidine, 4-(dimethylamino)pyridine, and dichloromethane.Deprotection: Camphorsulfonic acid
Camphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is a organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that exists as a colourless solid that is soluble in organic solvents....
, dicholoromethane, and methanol.
The secondary hydroxyl group in 1.8 was briefly protected during the protection of a tertiary hydroxyl group in the same compound.
TBS (tert-butyldimethylsilyl) [2]
Protection: Tert-butyldimethylsilyl triflate, lutidine, 4-(dimethylamino)pyridine, and dichloromethane.Deprotection: Camphorsulfonic acid
Camphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is a organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that exists as a colourless solid that is soluble in organic solvents....
Protection of the tertiary hydroxyl group in 1.8 was necessary to allow selective protection of other hydroxyl groups on the C ring.
TBS (tert-butyldimethylsilyl) [3]
Protection: Dichloromethane, imidazoleImidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
, and tert-butyldimethylsilyl chloride.
Deprotection: Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride or TBAF is a quaternary ammonium salt with the chemical formula 4N+F-. It is commercially available as the trihydrate and as a solution in tetrahydrofuran....
Protection of hydroxyl group in 3.4 allowed the ketone to undergo a Shapiro reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
to form the viyllithium compound 3.7.
TES (triethylsilyl) [1]
Protection: Triethylsilyl chloride and pyridinePyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
.
Deprotection: Hydrolysis using hydrofluoric acid
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
, pyridine and tetrahydrofuran.
Protection of the secondary hydroxyl group in 5.7 was necessary for the final addition of the tail to alcohol 7.2.
TES (triethylsilyl) [2]
Protection: See Ojima lactamOjima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
.
Deprotection: Hydrolysis using hydrofluoric acid
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
Protected secondary alcohol in Ojima lactam
Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
7.1 during reaction with alcohol 7.2 in the tail addition.
See also
- Paclitaxel total synthesis
- Danishefsky Taxol total synthesisDanishefsky Taxol total synthesisThe Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996...
- Holton Taxol total synthesisHolton Taxol total synthesisThe Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
- Kuwajima Taxol total synthesisKuwajima Taxol total synthesisThe Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s...
- Mukaiyama Taxol total synthesisMukaiyama Taxol total synthesisThe Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis....
- Wender Taxol total synthesisWender Taxol total synthesisThe Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul A. Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally...

