
Michaelis-Menten kinetics
Encyclopedia
In biochemistry
, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics
. It is named after German biochemist Leonor Michaelis
and Canadian physician Maud Menten
. The model takes the form of an equation describing the rate of enzymatic reactions, by relating reaction rate
 to
to 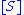 , the concentration
, the concentration
of a substrate S. Its formula is given by
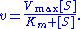
Here, represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations. The Michaelis constant
represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations. The Michaelis constant 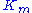 is the substrate concentration at which the reaction rate is half of
is the substrate concentration at which the reaction rate is half of  . Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions.
. Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions.
found that enzyme reactions were initiated by a bond between the enzyme and the substrate. His work was taken up by German biochemist Leonor Michaelis
and Canadian physician Maud Menten
who investigated the kinetics
of an enzymatic reaction mechanism, invertase
, that catalyzes the hydrolysis
of sucrose
into glucose
and fructose
. In 1913, they proposed a mathematical model of the reaction. It involves an enzyme
E binding to a substrate S to form a complex ES, which in turn is converted into a product
P and the enzyme. This may be represented schematically as

where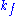 ,
, 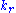 and
and 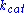 denote the rate constants, and the double arrows between S and ES represent the fact that enzyme-substrate binding is a reversible
denote the rate constants, and the double arrows between S and ES represent the fact that enzyme-substrate binding is a reversible
process.
Under certain assumptions – such as the enzyme concentration being much less than the substrate concentration – the rate of product formation is given by
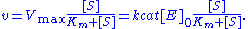
The reaction rate
increases with increasing substrate concentration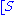 ], asymptotically
], asymptotically
approaching its maximum rate , attained when all enzyme is bound to substrate. It also follows that
, attained when all enzyme is bound to substrate. It also follows that 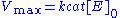 , where
, where 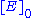 is the enzyme concentration.
is the enzyme concentration. 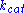 , the turnover number
, the turnover number
, is maximum number of substrate molecules converted to product per enzyme molecule per second.
The Michaelis constant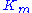 is the substrate concentration at which the reaction rate is at half-maximum, and is a measure of the substrate's affinity for the enzyme. A small
is the substrate concentration at which the reaction rate is at half-maximum, and is a measure of the substrate's affinity for the enzyme. A small 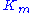 indicates high affinity, meaning that the rate will approach
indicates high affinity, meaning that the rate will approach  more quickly.
more quickly.
The model is used in a variety of biochemical situations other than enzyme-substrate interaction, including antigen-antibody binding
, DNA-DNA hybridization and protein-protein interaction
. It can be used to characterise a generic biochemical reaction, in the same way that the Langmuir equation
can be used to model generic adsorption
of biomolecular species.
The constant is a measure of how efficiently an enzyme converts a substrate into product. It has a theoretical upper limit of ; enzymes working close to this, such as fumarase, are termed superefficient.
is a measure of how efficiently an enzyme converts a substrate into product. It has a theoretical upper limit of ; enzymes working close to this, such as fumarase, are termed superefficient.
Michaelis-Menten kinetics have also been applied to a variety of spheres outside of biochemical reactions, including alveolar
clearance of dusts, the richness of species
pools, clearance of blood alcohol
, the photosynthesis-irradiance
relationship and bacterial phage
infection.
s that define the rate of change of reactants with time :
:
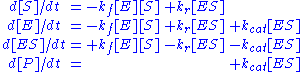
In this mechanism, the enzyme E is a catalyst, which only facilitates the reaction, so its total concentration, free plus combined,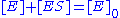 is a constant. This conservation law can also be obtained by adding the second and third equations above.
is a constant. This conservation law can also be obtained by adding the second and third equations above.
with the complex, and thus . Combining this relationship with the enzyme conservation law, the concentration of complex is
. Combining this relationship with the enzyme conservation law, the concentration of complex is
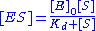
where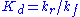 is the dissociation constant
is the dissociation constant
for the enzyme-substrate complex. Hence the velocity of the reaction – the rate at which P is formed – is
of the reaction – the rate at which P is formed – is
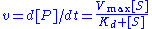
where is the maximum reaction velocity.
is the maximum reaction velocity.
and British geneticist J. B. S. Haldane
in 1925. They assumed that the concentration of the intermediate complex does not change on the time-scale of product formation – known as the quasi-steady-state
assumption or pseudo-steady-state-hypothesis. Mathematically, this assumption means . Combining this relationship with the enzyme conservation law, the concentration of complex is
. Combining this relationship with the enzyme conservation law, the concentration of complex is
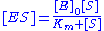
where
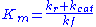
is known as the Michaelis constant. Hence the velocity of the reaction is
of the reaction is

. However, in the environment of a living cell where there is a high concentration of protein, the cytoplasm often behaves more like a gel than a liquid, limiting molecular movements and altering reaction rates. Whilst the law of mass action can be valid in heterogeneous environments, it is more appropriate to model the cytoplasm as a fractal
, in order to capture its limited-mobility kinetics.
The resulting reaction rates predicted by the two approaches are similar, with the only difference being that the equilibrium approximation defines the constant as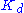 , whilst the quasi-steady-state approximation uses
, whilst the quasi-steady-state approximation uses 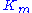 . However, each approach is founded upon a different assumption. The Michaelis-Menten equilibrium analysis is valid if the substrate reaches equilibrium on a much faster time-scale than the product is formed or, more precisely, that
. However, each approach is founded upon a different assumption. The Michaelis-Menten equilibrium analysis is valid if the substrate reaches equilibrium on a much faster time-scale than the product is formed or, more precisely, that
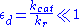
By contrast, the Briggs-Haldane quasi-steady-state analysis is valid if
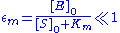
Thus it holds if the enzyme concentration is much less than the substrate concentration. Even if this is not satisfied, the approximation is valid if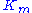 is large.
is large.
In both the Michaelis-Menten and Briggs-Haldane analyses, the quality of the approximation improves as decreases. However, in model building, Michaelis-Menten kinetics are often invoked without regard to the underlying assumptions.
decreases. However, in model building, Michaelis-Menten kinetics are often invoked without regard to the underlying assumptions.
 and
and 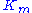 involves running a series of enzyme assay
involves running a series of enzyme assay
s at varying substrate concentrations , and measuring the initial reaction rate
, and measuring the initial reaction rate 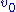 . 'Initial' here is taken to mean that the reaction rate is measured after a relatively short time period, during which it is assumed that the enzyme-substrate complex has formed, but that the substrate concentration held approximately constant, and so the equilibrium or quasi-steady-state approximation remain valid. By plotting reaction rate against concentration, and using nonlinear regression
. 'Initial' here is taken to mean that the reaction rate is measured after a relatively short time period, during which it is assumed that the enzyme-substrate complex has formed, but that the substrate concentration held approximately constant, and so the equilibrium or quasi-steady-state approximation remain valid. By plotting reaction rate against concentration, and using nonlinear regression
of the Michaelis-Menten equation, the parameters may be obtained.
Before computing facilities to perform nonlinear regression became available, graphical methods involving linearisation of the equation were used. A number of these were proposed, including the Eadie–Hofstee diagram, Hanes–Woolf plot and Lineweaver–Burk plot; of these, the Hanes-Woolf plot is the most accurate. However, while useful for visualization, all three methods distort the error structure of the data and are inferior to nonlinear regression. Nonetheless, their use can still be found in modern literature.
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
. It is named after German biochemist Leonor Michaelis
Leonor Michaelis
Leonor Michaelis was a German biochemist, physical chemist, and physician, known primarily for his work with Maud Menten on enzyme kinetics and Michaelis-Menten kinetics in 1913.-Early life and education:...
and Canadian physician Maud Menten
Maud Menten
Maud Leonora Menten was a Canadian medical scientist who made significant contributions to enzyme kinetics and histochemistry. Her name is associated with the famous Michaelis-Menten equation in biochemistry.Maud Menten was born in Port Lambton, Ontario and studied medicine at the University of...
. The model takes the form of an equation describing the rate of enzymatic reactions, by relating reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
 to
to 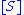 , the concentration
, the concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of a substrate S. Its formula is given by
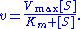
Here,
 represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations. The Michaelis constant
represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations. The Michaelis constant 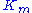 is the substrate concentration at which the reaction rate is half of
is the substrate concentration at which the reaction rate is half of  . Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions.
. Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions.Model
In 1903, French physical chemist Victor HenriVictor Henri
Victor Henri was a French physical chemist. He published over 500 papers in a variety of disciplines including biochemistry, physical chemistry, psychology and physiology....
found that enzyme reactions were initiated by a bond between the enzyme and the substrate. His work was taken up by German biochemist Leonor Michaelis
Leonor Michaelis
Leonor Michaelis was a German biochemist, physical chemist, and physician, known primarily for his work with Maud Menten on enzyme kinetics and Michaelis-Menten kinetics in 1913.-Early life and education:...
and Canadian physician Maud Menten
Maud Menten
Maud Leonora Menten was a Canadian medical scientist who made significant contributions to enzyme kinetics and histochemistry. Her name is associated with the famous Michaelis-Menten equation in biochemistry.Maud Menten was born in Port Lambton, Ontario and studied medicine at the University of...
who investigated the kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of an enzymatic reaction mechanism, invertase
Invertase
Invertase is an enzyme that catalyzes the hydrolysis of sucrose . The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose...
, that catalyzes the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of sucrose
Sucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
into glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
. In 1913, they proposed a mathematical model of the reaction. It involves an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
E binding to a substrate S to form a complex ES, which in turn is converted into a product
Product (biology)
In biochemistry, a product is something "manufactured" by an enzyme from its substrate. For example, the products of lactase are galactose and glucose, which are produced from the substrate lactose....
P and the enzyme. This may be represented schematically as

where
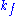 ,
, 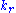 and
and 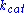 denote the rate constants, and the double arrows between S and ES represent the fact that enzyme-substrate binding is a reversible
denote the rate constants, and the double arrows between S and ES represent the fact that enzyme-substrate binding is a reversibleReversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
process.
Under certain assumptions – such as the enzyme concentration being much less than the substrate concentration – the rate of product formation is given by
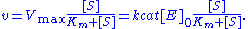
The reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
increases with increasing substrate concentration
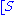 ], asymptotically
], asymptoticallyAsymptote
In analytic geometry, an asymptote of a curve is a line such that the distance between the curve and the line approaches zero as they tend to infinity. Some sources include the requirement that the curve may not cross the line infinitely often, but this is unusual for modern authors...
approaching its maximum rate
 , attained when all enzyme is bound to substrate. It also follows that
, attained when all enzyme is bound to substrate. It also follows that 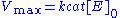 , where
, where 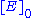 is the enzyme concentration.
is the enzyme concentration. 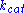 , the turnover number
, the turnover numberTurnover number
Turnover number has two related meanings:In enzymology, turnover number is defined as the maximum number of molecules of substrate that an enzyme can convert to product per catalytic site per unit of time and can be calculated as follows: kcat = Vmax/[E]T...
, is maximum number of substrate molecules converted to product per enzyme molecule per second.
The Michaelis constant
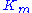 is the substrate concentration at which the reaction rate is at half-maximum, and is a measure of the substrate's affinity for the enzyme. A small
is the substrate concentration at which the reaction rate is at half-maximum, and is a measure of the substrate's affinity for the enzyme. A small 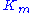 indicates high affinity, meaning that the rate will approach
indicates high affinity, meaning that the rate will approach  more quickly.
more quickly.The model is used in a variety of biochemical situations other than enzyme-substrate interaction, including antigen-antibody binding
Immune complex
An immune complex is formed from the integral binding of an antibody to a soluble antigen. The bound antigen acting as a specific epitope, bound to an antibody is referred to as a singular immune complex....
, DNA-DNA hybridization and protein-protein interaction
Protein-protein interaction
Protein–protein interactions occur when two or more proteins bind together, often to carry out their biological function. Many of the most important molecular processes in the cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein...
. It can be used to characterise a generic biochemical reaction, in the same way that the Langmuir equation
Langmuir equation
The Langmuir equation relates the coverage or adsorption of molecules on a solid surface to gas pressure or concentration of a medium above the solid surface at a fixed temperature. The equation was developed by Irving Langmuir in 1916...
can be used to model generic adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
of biomolecular species.
Applications
Parameter values vary wildly between enzymes:| Enzyme |  (M) (M) |  (1/s) (1/s) | 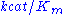 (1/M.s) (1/M.s) |
|---|---|---|---|
| Chymotrypsin Chymotrypsin Chymotrypsin is a digestive enzyme that can perform proteolysis. Chymotrypsin preferentially cleaves peptide amide bonds where the carboxyl side of the amide bond is a tyrosine, tryptophan, or phenylalanine. These amino acids contain an aromatic ring in their sidechain that fits into a... |
1.5 × 10-2 | 0.14 | 9.3 |
| Pepsin Pepsin Pepsin is an enzyme whose precursor form is released by the chief cells in the stomach and that degrades food proteins into peptides. It was discovered in 1836 by Theodor Schwann who also coined its name from the Greek word pepsis, meaning digestion... |
3.0 × 10-4 | 0.50 | 1.7 × 103 |
| Tyrosyl-tRNA synthetase | 9.0 × 10-4 | 7.6 | 8.4 × 103 |
| Ribonuclease Ribonuclease Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.-Function:All organisms studied contain... |
7.9 × 10-3 | 7.9 × 102 | 1.0 × 105 |
| Carbonic anhydrase Carbonic anhydrase The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst... |
2.6 × 10-2 | 4.0 × 105 | 1.5 × 107 |
| Fumarase Fumarase Fumarase is an enzyme that catalyzes the reversible hydration/dehydration of Fumarate to malate. Fumarase comes in two forms: mitochondrial and cytosolic... |
5.0 × 10-6 | 8.0 × 102 | 1.6 × 108 |
The constant
 is a measure of how efficiently an enzyme converts a substrate into product. It has a theoretical upper limit of ; enzymes working close to this, such as fumarase, are termed superefficient.
is a measure of how efficiently an enzyme converts a substrate into product. It has a theoretical upper limit of ; enzymes working close to this, such as fumarase, are termed superefficient.Michaelis-Menten kinetics have also been applied to a variety of spheres outside of biochemical reactions, including alveolar
Pulmonary alveolus
An alveolus is an anatomical structure that has the form of a hollow cavity. Found in the lung parenchyma, the pulmonary alveoli are the dead ends of the respiratory tree, which outcrop from either alveolar sacs or alveolar ducts, which are both sites of gas exchange with the blood as well...
clearance of dusts, the richness of species
Species richness
Species richness is the number of different species in a given area. It is represented in equation form as S.Species richness is the fundamental unit in which to assess the homogeneity of an environment. Typically, species richness is used in conservation studies to determine the sensitivity of...
pools, clearance of blood alcohol
Blood alcohol content
Blood alcohol content , also called blood alcohol concentration, blood ethanol concentration, or blood alcohol level is most commonly used as a metric of alcohol intoxication for legal or medical purposes....
, the photosynthesis-irradiance
PI Curve
The PI Curve is a graphical representation of the empirical relationship between solar irradiance and photosynthesis. A derivation of the Michaelis-Menten curve, it shows the generally positive correlation between light intensity and photosynthetic rate...
relationship and bacterial phage
Bacteriophage
A bacteriophage is any one of a number of viruses that infect bacteria. They do this by injecting genetic material, which they carry enclosed in an outer protein capsid...
infection.
Derivation
Applying the law of mass action, which states that the rate of a reaction is proportional to the product of the concentrations of the reactants, gives a system of four non-linear ordinary differential equationOrdinary differential equation
In mathematics, an ordinary differential equation is a relation that contains functions of only one independent variable, and one or more of their derivatives with respect to that variable....
s that define the rate of change of reactants with time
 :
: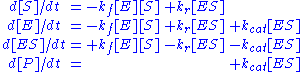
In this mechanism, the enzyme E is a catalyst, which only facilitates the reaction, so its total concentration, free plus combined,
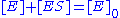 is a constant. This conservation law can also be obtained by adding the second and third equations above.
is a constant. This conservation law can also be obtained by adding the second and third equations above.Equilibrium approximation
In their original analysis, Michaelis and Menten assumed that the substrate is in instantaneous chemical equilibriumChemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with the complex, and thus
 . Combining this relationship with the enzyme conservation law, the concentration of complex is
. Combining this relationship with the enzyme conservation law, the concentration of complex is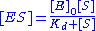
where
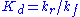 is the dissociation constant
is the dissociation constantDissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
for the enzyme-substrate complex. Hence the velocity
 of the reaction – the rate at which P is formed – is
of the reaction – the rate at which P is formed – is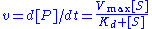
where
 is the maximum reaction velocity.
is the maximum reaction velocity.Quasi-steady-state approximation
An alternative analysis of the system was undertaken by British botanist G. E. BriggsGeorge Edward Briggs
George Edward Briggs was a British botanist.He was born in Grimsby, Lincolnshire, the eldest son of Walker Thomas and Susan Briggs....
and British geneticist J. B. S. Haldane
J. B. S. Haldane
John Burdon Sanderson Haldane FRS , known as Jack , was a British-born geneticist and evolutionary biologist. A staunch Marxist, he was critical of Britain's role in the Suez Crisis, and chose to leave Oxford and moved to India and became an Indian citizen...
in 1925. They assumed that the concentration of the intermediate complex does not change on the time-scale of product formation – known as the quasi-steady-state
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
assumption or pseudo-steady-state-hypothesis. Mathematically, this assumption means
 . Combining this relationship with the enzyme conservation law, the concentration of complex is
. Combining this relationship with the enzyme conservation law, the concentration of complex is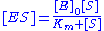
where
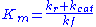
is known as the Michaelis constant. Hence the velocity
 of the reaction is
of the reaction is
Assumptions and limitations
The first step in the derivation applies the law of mass action, which is reliant on free diffusionDiffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
. However, in the environment of a living cell where there is a high concentration of protein, the cytoplasm often behaves more like a gel than a liquid, limiting molecular movements and altering reaction rates. Whilst the law of mass action can be valid in heterogeneous environments, it is more appropriate to model the cytoplasm as a fractal
Fractal
A fractal has been defined as "a rough or fragmented geometric shape that can be split into parts, each of which is a reduced-size copy of the whole," a property called self-similarity...
, in order to capture its limited-mobility kinetics.
The resulting reaction rates predicted by the two approaches are similar, with the only difference being that the equilibrium approximation defines the constant as
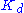 , whilst the quasi-steady-state approximation uses
, whilst the quasi-steady-state approximation uses 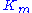 . However, each approach is founded upon a different assumption. The Michaelis-Menten equilibrium analysis is valid if the substrate reaches equilibrium on a much faster time-scale than the product is formed or, more precisely, that
. However, each approach is founded upon a different assumption. The Michaelis-Menten equilibrium analysis is valid if the substrate reaches equilibrium on a much faster time-scale than the product is formed or, more precisely, that 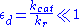
By contrast, the Briggs-Haldane quasi-steady-state analysis is valid if
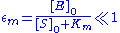
Thus it holds if the enzyme concentration is much less than the substrate concentration. Even if this is not satisfied, the approximation is valid if
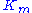 is large.
is large.In both the Michaelis-Menten and Briggs-Haldane analyses, the quality of the approximation improves as
 decreases. However, in model building, Michaelis-Menten kinetics are often invoked without regard to the underlying assumptions.
decreases. However, in model building, Michaelis-Menten kinetics are often invoked without regard to the underlying assumptions.Determination of constants
The typical method for determining the constants and
and 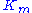 involves running a series of enzyme assay
involves running a series of enzyme assayEnzyme assay
Enzyme assays are laboratory methods for measuring enzymatic activity. They are vital for the study of enzyme kinetics and enzyme inhibition.-Enzyme units:...
s at varying substrate concentrations
 , and measuring the initial reaction rate
, and measuring the initial reaction rate 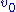 . 'Initial' here is taken to mean that the reaction rate is measured after a relatively short time period, during which it is assumed that the enzyme-substrate complex has formed, but that the substrate concentration held approximately constant, and so the equilibrium or quasi-steady-state approximation remain valid. By plotting reaction rate against concentration, and using nonlinear regression
. 'Initial' here is taken to mean that the reaction rate is measured after a relatively short time period, during which it is assumed that the enzyme-substrate complex has formed, but that the substrate concentration held approximately constant, and so the equilibrium or quasi-steady-state approximation remain valid. By plotting reaction rate against concentration, and using nonlinear regressionNonlinear regression
In statistics, nonlinear regression is a form of regression analysis in which observational data are modeled by a function which is a nonlinear combination of the model parameters and depends on one or more independent variables...
of the Michaelis-Menten equation, the parameters may be obtained.
Before computing facilities to perform nonlinear regression became available, graphical methods involving linearisation of the equation were used. A number of these were proposed, including the Eadie–Hofstee diagram, Hanes–Woolf plot and Lineweaver–Burk plot; of these, the Hanes-Woolf plot is the most accurate. However, while useful for visualization, all three methods distort the error structure of the data and are inferior to nonlinear regression. Nonetheless, their use can still be found in modern literature.

