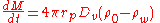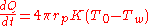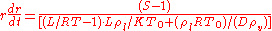Mason equation
Encyclopedia
The Mason equation is an approximate analytical expression for the growth (due to condensation
) or evaporation
of a water droplet—it is due to the meteorologist B. J. Mason
. The expression is found by recognising that mass diffusion
towards the water drop in a supersaturated environment transports energy as latent heat
, and this has to be balanced by the diffusion of sensible heat
back across the boundary layer
, (and the energy of heatup of the drop, but for a cloud-sized drop this last term is usually small).
; the two energy transport terms must be nearly equal but opposite in sign and so this sets the interface temperature of the drop. The resulting expression for the growth rate is significantly lower than that expected if the drop were not warmed by the latent heat.
Thus if the drop has a size r, the inward mass flow rate is given by
and the sensible heat flux
by
and the final expression for the growth rate is
where
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
) or evaporation
Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
of a water droplet—it is due to the meteorologist B. J. Mason
Basil John Mason
Sir John Mason, CB, FRCP, FRCPEd, FRFPS, FRS is an expert on cloud physics and former Director of the UK Meteorological Office.His work includes the Mason Equation, giving the growth or evaporation of small water droplets...
. The expression is found by recognising that mass diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
towards the water drop in a supersaturated environment transports energy as latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
, and this has to be balanced by the diffusion of sensible heat
Sensible heat
Sensible heat is the energy exchanged by a thermodynamic system that has as its sole effect a change of temperature.The term is used in contrast to a latent heat, which is the amount of energy exchanged that is hidden, meaning it cannot be observed as a change of temperature...
back across the boundary layer
Boundary layer
In physics and fluid mechanics, a boundary layer is that layer of fluid in the immediate vicinity of a bounding surface where effects of viscosity of the fluid are considered in detail. In the Earth's atmosphere, the planetary boundary layer is the air layer near the ground affected by diurnal...
, (and the energy of heatup of the drop, but for a cloud-sized drop this last term is usually small).
Equation
In Mason's formulation the changes in temperature across the boundary layer can be related to the changes in saturated vapour pressure by the Clausius-Clapeyron relationClausius-Clapeyron relation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, who defined it sometime after 1834, is a way of characterizing a discontinuous phase transition between two phases of matter. On a pressure–temperature diagram, the line separating the two phases is known...
; the two energy transport terms must be nearly equal but opposite in sign and so this sets the interface temperature of the drop. The resulting expression for the growth rate is significantly lower than that expected if the drop were not warmed by the latent heat.
Thus if the drop has a size r, the inward mass flow rate is given by
and the sensible heat flux
Heat flux
Heat flux or thermal flux is the rate of heat energy transfer through a given surface. The SI derived unit of heat rate is joule per second, or watt. Heat flux is the heat rate per unit area. In SI units, heat flux is measured in W/m2]. Heat rate is a scalar quantity, while heat flux is a vectorial...
by
and the final expression for the growth rate is
where
- S is the supersaturationSupersaturationThe term supersaturation refers to a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances...
far from the drop - L is the latent heatLatent heatLatent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
- K is the vapour thermal conductivityThermal conductivityIn physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
- D is the binary diffusion coefficient
- R is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...