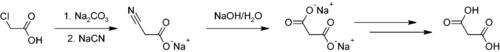Malonic acid
Encyclopedia
Malonic acid is a dicarboxylic acid
with structure C
H
2(COOH)2. The ion
ised form of malonic acid, as well as its ester
s and salt
s, are known as malonate
s. For example, diethyl malonate
is malonic acid's ethyl
ester
. The name originates from the Greek
word μᾶλον (malon) meaning 'apple'.
salt of malonic acid occurs in high concentrations in beetroot
. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain
.
:
Sodium carbonate
generates the sodium salt, which is then reacted with sodium cyanide
to provide the cyano acetic acid salt via a nucleophilic substitution
. The nitrile
group can be hydrolyzed
with sodium hydroxide to sodium malonate, and acidification affords malonic acid.
with urea
to form barbituric acid
. Malonic acid is also frequently used as an enolate in Knoevenagel condensation
s or condensed with acetone
to form Meldrum's acid
. The esters of malonic acid are also used as a -CH2COOH synthon
in the malonic ester synthesis
.
Dicarboxylic acid
Dicarboxylic acids are organic compounds that contain two carboxylic acid functional groups. In molecular formulae for dicarboxylic acids, these groups are often written as HOOC-R-COOH, where R may be an alkyl, alkenyl, alkynyl, or aryl group...
with structure C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
2(COOH)2. The ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
ised form of malonic acid, as well as its ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s and salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
s, are known as malonate
Malonate
The malonate or propanedioate ion is CH222− . Malonate compounds include salts and esters of malonic acid, such as*diethyl malonate, 2,*dimethyl malonate, 2,...
s. For example, diethyl malonate
Diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes...
is malonic acid's ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
. The name originates from the Greek
Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
word μᾶλον (malon) meaning 'apple'.
Biochemistry
The calciumCalcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
salt of malonic acid occurs in high concentrations in beetroot
Beetroot
The beetroot, also known as the table beet, garden beet, red beet or informally simply as beet, is one of the many cultivated varieties of beets and arguably the most commonly encountered variety in North America, Central America and Britain.-Consumption:The usually deep-red roots of beetroot are...
. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
.
Preparation
A classical preparation of malonic acid starts from chloroacetic acidChloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building-block in organic synthesis.-Production:...
:
Sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
generates the sodium salt, which is then reacted with sodium cyanide
Sodium cyanide
Sodium cyanide is an inorganic compound with the formula NaCN. This highly toxic colorless salt is used mainly in gold mining but has other niche applications...
to provide the cyano acetic acid salt via a nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
. The nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
group can be hydrolyzed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
with sodium hydroxide to sodium malonate, and acidification affords malonic acid.
Organic reactions
In a well-known reaction, malonic acid condensesCondensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
with urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
to form barbituric acid
Barbituric acid
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active...
. Malonic acid is also frequently used as an enolate in Knoevenagel condensation
Knoevenagel condensation
The Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the Aldol condensation.A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule...
s or condensed with acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
to form Meldrum's acid
Meldrum's acid
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid. Meldrum misidentified the structure as a β-lactone of...
. The esters of malonic acid are also used as a -CH2COOH synthon
Synthon
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
in the malonic ester synthesis
Malonic ester synthesis
The malonic ester synthesis is a chemical reaction where diethyl malonate or another ester of malonic acid is alkylated at the carbon alpha to both carbonyl groups, and then converted to a substituted acetic acid. The major drawback of malonic ester synthesis is that the alkylation stage can also...
.