
Lower sulfur oxides
Encyclopedia

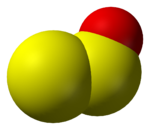
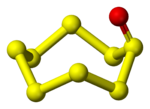
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s are a group of chemical compounds consisting of :
- SO, sulfur monoxideSulfur monoxideSulfur monoxide is an inorganic compound with formula . It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 . It has been detected in space but is rarely encountered intact otherwise.-Structure and bonding:The SO molecule has a triplet ground state similar...
and its dimer S2O2 - S2O
- sulfur monoxides, SnO, based on cyclic Sn rings where n is 5-10
- S7O2
- polymeric oxides, polysulfuroxides sometimes termed PSO's
Interest in the lower sulfur oxides has increased because of the need to understand terrestrial atmospheric sulfur pollution and the finding that the extraterrestrial atmospheres of Io
Io (moon)
Io ) is the innermost of the four Galilean moons of the planet Jupiter and, with a diameter of , the fourth-largest moon in the Solar System. It was named after the mythological character of Io, a priestess of Hera who became one of the lovers of Zeus....
, one of Jupiter
Jupiter
Jupiter is the fifth planet from the Sun and the largest planet within the Solar System. It is a gas giant with mass one-thousandth that of the Sun but is two and a half times the mass of all the other planets in our Solar System combined. Jupiter is classified as a gas giant along with Saturn,...
s moons, and Venus
Venus
Venus is the second planet from the Sun, orbiting it every 224.7 Earth days. The planet is named after Venus, the Roman goddess of love and beauty. After the Moon, it is the brightest natural object in the night sky, reaching an apparent magnitude of −4.6, bright enough to cast shadows...
contain significant amounts of sulfur oxides.
Some compounds reported by early workers such as the blue "sesquioxide", S2O3, formed by dissolving sulfur in liquid SO3 appears to be a mixture of polysulfate salts of the S42+ and S82+ ions.
Sulfur monoxide
Sulfur monoxide
Sulfur monoxide is an inorganic compound with formula . It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 . It has been detected in space but is rarely encountered intact otherwise.-Structure and bonding:The SO molecule has a triplet ground state similar...
, monomer (SO) and dimer (S2O2)
- These are stable molecules that have been trapped at low temperature (see sulfur monoxide articleSulfur monoxideSulfur monoxide is an inorganic compound with formula . It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 . It has been detected in space but is rarely encountered intact otherwise.-Structure and bonding:The SO molecule has a triplet ground state similar...
).
Disulfur monoxide
Disulfur monoxide
Disulfur monoxide or sulfur suboxide is an unstable gas being a compound of sulfur and oxygen with formula S2O. It is one of the lower sulfur oxides. It is a colourless gas. It can be formed by reacting thionyl chloride with silver sulfide:...
, S2O
- S2O has a non linear structure, like sulfur dioxideSulfur dioxideSulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
, SO2, ozoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
, O3 and S3. The S-S bond is 188.4pm, the S-O bond is 146.5pm and the SSO angle is 117.88. The two dipole moment components are μa = 0.875D and μb = 1.18D
Trisulfur monoxide, S3O
- This unstable neutral molecule has been found in the gas phase using neutralization-reionization mass spectrometry. Both ring and chain structures were found.
SnO
- A number of monoxides SnO are known where n= 5-10, where the oxygen is bonded to one member of the Sn sulfur ring. They can be prepared by oxidising the related cyclo-Sn elemental sulfur rings (these are allotropes of sulfurAllotropes of sulfurThere are a large number of allotropes of sulfur. In this respect, sulfur is second only to carbon.The most common form found in nature is yellow orthorhombic α-sulfur, which contains puckered rings of . Chemistry students may have seen "plastic sulfur"; this is not an allotrope but a mixture of...
) with trifluoroperoxoacetic acid, CF3C(O)OOH. The compounds are all dark coloured and decompose to give sulfur and sulfur dioxide.
S6O2, S7O2
- S6O2 and S7O2 can be prepared by oxidising cyclo-S6 and cyclo-S7 respectively, with trifluoroperoxoacetic acid, although crystalline S6O2 has not been isolated.
Polymeric sulfuroxides
- These have been studied to determine whether they are a factor in the observed colour of IoIo (moon)Io ) is the innermost of the four Galilean moons of the planet Jupiter and, with a diameter of , the fourth-largest moon in the Solar System. It was named after the mythological character of Io, a priestess of Hera who became one of the lovers of Zeus....
.

