
List of thermodynamic properties
Encyclopedia
{| class="wikitable" style="text-align:center"
|+Thermodynamic properties
and their characteristics
!Property!!Symbol!!Units!!Extensive?
!!Intensive?
!!Conjugate
!!Potential?!!State
qty.
?!!Process
qty.
?
|-
|style="text-align:left"|Activity
|
|
|
|
|
|
|
|
|-
|style="text-align:left"|Altitude
|
|m
|
|
|
|
|
|
|-
|style="text-align:left"|Chemical potential
|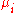
|kJ/mol
|
|
|Particle
number
|
|
|
|-
|style="text-align:left"|Compressibility (adiabatic)
| ,
, 
|Pa−1
|
|
|
|
|
|
|-
|style="text-align:left"|Compressibility (isothermal)
|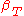 ,
, 
|Pa−1
|
|
|
|
|
|
|-
|style="text-align:left"|Cryoscopic constant
|
|K·kg/mol
|
|
|
|
|
|
|-
|style="text-align:left"|Density
|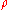
|kg/m3
|
|
|
|
|
|
|-
|style="text-align:left"|Ebullioscopic constant
|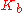
|
|
|
|
|
|
|
|-
|style="text-align:left"|Enthalpy
|
|J
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific enthalpy
|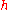
|J/kg
|
|
|
|
|
|
|-
|style="text-align:left"|Entropy
|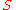
|J/K
|
|
|Temperature
| (entropic
)
|
|
|-
|style="text-align:left"|↳ Specific entropy
|
|J/(kg K)
|
|
|
|
|
|
|-
|style="text-align:left"|Fugacity
|
|N/m²
|
|
|
|
|
|
|-
|style="text-align:left"|Gas constant
|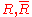
|J/K
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific gas constant
(for a particular substance)
|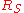
|J/(kg K)
|
|
|
|
|
|
|-
|style="text-align:left"|Gibbs free energy
|
|J
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific Gibbs free entropy
|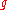
|J/(kg K)
|
|
|
|
|
|
|-
|style="text-align:left"|Gibbs free entropy
|
|J/K
|
|
|
| (entropic
)
|
|
|-
|style="text-align:left"|Grand / Landau potential
|
|J
|
|
|
|
|
|
|-
|style="text-align:left"|Heat
|
|J
|
|
|
|
|
|
|-
|style="text-align:left"|Heat capacity
(constant pressure)
|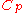
|J/K
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific heat capacity
(constant pressure)
|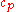
|J/(kg·K)
|
|
|
|
|
|
|-
|style="text-align:left"|Heat capacity
(constant volume)
|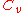
|J/K
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific heat capacity
(constant volume)
|
|J/(kg·K)
|
|
|
|
|
|
|-
|style="text-align:left"|Helmholtz free energy
| ,
, 
|J
|
|
|
|
|
|
|-
|style="text-align:left"|Helmholtz free entropy
|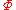
|J/K
|
|
|
| (entropic
)
|
|
|-
|style="text-align:left"|Internal energy
|
|J
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific internal energy
|
|J/kg
|
|
|
|
|
|
|-
|style="text-align:left"|Internal pressure
|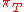
|Pa
|
|
|
|
|
|
|-
|style="text-align:left"|Mass
|
|kg
|
|
|
|
|
|
|-
|style="text-align:left"|Particle number
|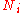
|
|
|
|Chemical
potential
|
|
|
|-
|style="text-align:left"|Pressure
|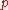
|Pa
|
|
|Volume
|
|
|
|-
|style="text-align:left"|Temperature
|
|K
|
|
|Entropy
|
|
|
|-
|style="text-align:left"|Thermal conductivity
|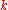
|W/(m·K)
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal diffusivity
|
|m²/s
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal expansion
(linear)
|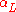
|K−1
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal expansion
(area)
|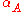
|K−1
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal expansion
(volumetric)
|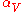
|K−1
|
|
|
|
|
|
|-
|style="text-align:left"|Vapor quality
|
|
|
|
|
|
|
|
|-
|style="text-align:left"|Volume
|
|m3
|
|
|Pressure
|
|
|
|-
|style="text-align:left"|↳ Specific volume
|
|m3/kg
|
|
|
|
|
|
|-
Specific properties are expressed on a per mass basis; in some circumstances other dimensions could be used, such as per-mole.
|+Thermodynamic properties
Physical property
A physical property is any property that is measurable whose value describes a physical system's state. The changes in the physical properties of a system can be used to describe its transformations ....
and their characteristics
!Property!!Symbol!!Units!!Extensive?
Intensive and extensive properties
In the physical sciences, an intensive property , is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.By contrast, an extensive property In the physical sciences, an intensive property (also called a bulk...
!!Intensive?
Intensive and extensive properties
In the physical sciences, an intensive property , is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.By contrast, an extensive property In the physical sciences, an intensive property (also called a bulk...
!!Conjugate
Conjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
!!Potential?!!State
qty.
State function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
?!!Process
qty.
Process function
A process function, process quantity, or a path function is a physical quantity that describes the transition of a system from an equilibrium state to another equilibrium state. As an example, mechanical work and heat are process quantities because they describe quantitatively the transition...
?
|-
|style="text-align:left"|Activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
|

|
|
|
|
|
|
|
|-
|style="text-align:left"|Altitude
Altitude
Altitude or height is defined based on the context in which it is used . As a general definition, altitude is a distance measurement, usually in the vertical or "up" direction, between a reference datum and a point or object. The reference datum also often varies according to the context...
|
|m
|
|
|
|
|
|
|-
|style="text-align:left"|Chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
|
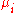
|kJ/mol
|
|
|Particle
number
|
|
|
|-
|style="text-align:left"|Compressibility (adiabatic)
|
 ,
, 
|Pa−1
|
|
|
|
|
|
|-
|style="text-align:left"|Compressibility (isothermal)
|
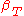 ,
, 
|Pa−1
|
|
|
|
|
|
|-
|style="text-align:left"|Cryoscopic constant
Cryoscopic constant
In thermodynamics, the cryoscopic constant, Kf, allows one to relate molality to freezing point depression. It is the ratio of the latter to the former:\triangle T_f = K_f \cdot m \cdot i...
|

|K·kg/mol
|
|
|
|
|
|
|-
|style="text-align:left"|Density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
|
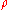
|kg/m3
|
|
|
|
|
|
|-
|style="text-align:left"|Ebullioscopic constant
Ebullioscopic constant
In thermodynamics, the ebullioscopic constant, Kb, allows one to relate molality to boiling point elevation. It is the ratio of the latter to the former:\Delta T = i\cdot K_b \cdot mi is the Vant Hoff factor...
|
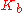
|
|
|
|
|
|
|
|-
|style="text-align:left"|Enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
|

|J
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific enthalpy
|
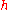
|J/kg
|
|
|
|
|
|
|-
|style="text-align:left"|Entropy
|
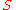
|J/K
|
|
|Temperature
| (entropic
Free entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu-Planck potentials , or free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function...
)
|
|
|-
|style="text-align:left"|↳ Specific entropy
|

|J/(kg K)
|
|
|
|
|
|
|-
|style="text-align:left"|Fugacity
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
|

|N/m²
|
|
|
|
|
|
|-
|style="text-align:left"|Gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
|
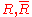
|J/K
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific gas constant
(for a particular substance)
|
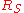
|J/(kg K)
|
|
|
|
|
|
|-
|style="text-align:left"|Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
|

|J
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific Gibbs free entropy
|
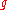
|J/(kg K)
|
|
|
|
|
|
|-
|style="text-align:left"|Gibbs free entropy
Free entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu-Planck potentials , or free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function...
|

|J/K
|
|
|
| (entropic
Free entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu-Planck potentials , or free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function...
)
|
|
|-
|style="text-align:left"|Grand / Landau potential
|

|J
|
|
|
|
|
|
|-
|style="text-align:left"|Heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
|

|J
|
|
|
|
|
|
|-
|style="text-align:left"|Heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
(constant pressure)
|
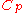
|J/K
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific heat capacity
(constant pressure)
|
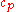
|J/(kg·K)
|
|
|
|
|
|
|-
|style="text-align:left"|Heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
(constant volume)
|
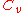
|J/K
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific heat capacity
(constant volume)
|

|J/(kg·K)
|
|
|
|
|
|
|-
|style="text-align:left"|Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
|
 ,
, 
|J
|
|
|
|
|
|
|-
|style="text-align:left"|Helmholtz free entropy
Free entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu-Planck potentials , or free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function...
|
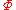
|J/K
|
|
|
| (entropic
Free entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu-Planck potentials , or free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function...
)
|
|
|-
|style="text-align:left"|Internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
|

|J
|
|
|
|
|
|
|-
|style="text-align:left"|↳ Specific internal energy
|

|J/kg
|
|
|
|
|
|
|-
|style="text-align:left"|Internal pressure
Internal pressure
Internal pressure is a measure of how the internal energy of a system changes when it expands or contracts at constant temperature. It has the same dimensions as pressure, the SI unit of which is 1 pascal.Internal pressure is usually given the symbol \pi_T...
|
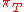
|Pa
|
|
|
|
|
|
|-
|style="text-align:left"|Mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
|

|kg
|
|
|
|
|
|
|-
|style="text-align:left"|Particle number
Particle number
The particle number of a thermodynamic system, conventionally indicated with the letter N, is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle...
|
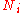
|
|
|
|Chemical
potential
|
|
|
|-
|style="text-align:left"|Pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
|
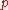
|Pa
|
|
|Volume
|
|
|
|-
|style="text-align:left"|Temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
|

|K
|
|
|Entropy
|
|
|
|-
|style="text-align:left"|Thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
|
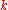
|W/(m·K)
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal diffusivity
Thermal diffusivity
In heat transfer analysis, thermal diffusivity is the thermal conductivity divided by density and specific heat capacity at constant pressure. It has the SI unit of m²/s...
|

|m²/s
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
(linear)
|
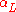
|K−1
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
(area)
|
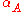
|K−1
|
|
|
|
|
|
|-
|style="text-align:left"|Thermal expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
(volumetric)
|
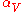
|K−1
|
|
|
|
|
|
|-
|style="text-align:left"|Vapor quality
Vapor quality
In thermodynamics, vapor quality is a quantitative description of the usefulness of a vapor to do mechanical work. The quality of a fluid is the percentage of mass that is vapor; i.e. saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%...
|

|
|
|
|
|
|
|
|-
|style="text-align:left"|Volume
|

|m3
|
|
|Pressure
|
|
|
|-
|style="text-align:left"|↳ Specific volume
Specific volume
In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass. It is the reciprocal of density:In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass...
|

|m3/kg
|
|
|
|
|
|
|-
Specific properties are expressed on a per mass basis; in some circumstances other dimensions could be used, such as per-mole.
See also
- Dimensionless numbers
- Thermodynamic databases for pure substancesThermodynamic databases for pure substancesThermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are collected as tables or are calculated from thermodynamic datafiles...
- Thermodynamic variable

