
Liquid-liquid extraction
Encyclopedia
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities
in two different immiscible
liquids, usually water and an organic solvent
. It is an extraction of a substance from one liquid phase into another liquid phase. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a separatory funnel
. This type of process is commonly performed after a chemical reaction as part of the work-up.
The term partitioning is commonly used to refer to the underlying chemical and physical processes involved in liquid–liquid extraction but may be fully synonymous. The term solvent extraction can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.
Solvent extraction is used in nuclear reprocessing
, ore processing, the production of fine organic compound
s, the processing of perfume
s, the production of vegetable oils
and biodiesel
, and other industries.
Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salt
s, metals can be extracted from one phase to the other. This is related to a mercury
electrode
where a metal can be reduced, the metal will often then dissolve in the mercury to form an amalgam
that modifies its electrochemistry greatly. For example, it is possible for sodium
cations to be reduced at a mercury cathode
to form sodium amalgam
, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A detergent
or fine solid
can be used to stabilize an emulsion
, or third phase
.
of a solute
in the organic phase divided by its concentration in the aqueous phase. Depending on the system, the distribution ratio can be a function of temperature, the concentration of chemical species in the system, and a large number of other parameters.
Note that D is related to the ΔG of the extraction process.
Sometimes, the distribution ratio is referred to as the partition coefficient
, which is often expressed as the logarithm
. Note that a distribution ratio for uranium
and neptunium
between two inorganic solids (zirconolite
and perovskite
) has been reported.
In solvent extraction, two immiscible liquids are shaken together. The more polar
solutes dissolve preferentially in the more polar solvent, and the less polar solutes in the less polar solvent. In this experiment, the nonpolar halogens preferentially dissolve in the nonpolar mineral oil.
(DNi) is 10 and the distribution ratio for silver
(DAg) is 100, then the silver/nickel separation factor (SFAg/Ni) is equal to DAg/DNi = SFAg/Ni = 10.
to indium
, and the product is a 1:99 mixture of cadmium
and indium
, then the decontamination factor (for the removal of cadmium) of the process is 0.1 / 0.01 = 10.
from a mixture
of water
and 5% acetic acid
using ether
, then the anisole will enter the organic phase. The two phases would then be separated.
The acetic acid can then be scrubbed (removed) from the organic phase by shaking the organic extract with sodium bicarbonate
. The acetic acid reacts with the sodium bicarbonate
to form sodium acetate
, carbon dioxide
, and water
.
for the processing of metals such as the lanthanides; because the separation factors between the lanthanides are so small many extraction stages are needed. In the multistage processes, the aqueous raffinate
from one extraction unit is fed to the next unit as the aqueous feed, while the organic phase is moved in the opposite direction. Hence, in this way, even if the separation between two metals in each stage is small, the overall system can have a higher decontamination factor.
Multistage countercurrent
arrays have been used for the separation of lanthanides. For the design of a good process, the distribution ratio should be not too high (>100) or too low (<0.1) in the extraction portion of the process. It is often the case that the process will have a section for scrubbing unwanted metals from the organic phase, and finally a stripping
section to obtain the metal back from the organic phase.
Multistage Podbielniak contactor centrifuges produce three to five stages of theoretical extraction in a single countercurrent pass, and are used in fermentation-based pharmaceutical and food additive production facilities.
es can be extracted from one phase to another without the need for a chemical reaction (see absorption). This is the simplest type of solvent extraction. When a solvent is extracted, two immiscible liquids are shaken together. The more polar solutes dissolve preferentially in the more polar solvent, and the less polar solutes in the less polar solvent. Some solutes that do not at first sight appear to undergo a reaction during the extraction process do not have distribution ratio that is independent of concentration. A classic example is the extraction of carboxylic acids (HA) into nonpolar media such as benzene
. Here, it is often the case that the carboxylic acid will form a dimer in the organic layer so the distribution ratio will change as a function of the acid concentration (measured in either phase).
For this case, the extraction constant k is described by k = HAorganic2/HAaqueous
, plutonium
, or thorium
from acid solutions. One solvent used for this purpose is the organophosphate
tri-n-butyl phosphate. The PUREX
process that is commonly used in nuclear reprocessing
uses a mixture of tri-n-butyl phosphate and an inert
hydrocarbon
(kerosene
), the uranium(VI) are extracted from strong nitric acid and are back-extracted (stripped) using weak nitric acid. An organic soluble uranium complex
[UO2(TBP)2(NO3)2] is formed, then the organic layer bearing the uranium is brought into contact with a dilute
nitric acid solution; the equilibrium is shifted away from the organic soluble uranium complex and towards the free TBP and uranyl nitrate
in dilute nitric acid. The plutonium(IV) forms a similar complex to the uranium(VI), but it is possible to strip the plutonium in more than one way; a reducing agent
that converts the plutonium
to the trivalent oxidation state
can be added. This oxidation state
does not form a stable complex with TBP and nitrate
unless the nitrate concentration is very high (circa 10 mol/L nitrate is required in the aqueous phase). Another method is to simply use dilute nitric acid as a stripping agent for the plutonium. This PUREX chemistry is a classic example of a solvation
extraction.
Here in this case DU = k TBP
2NO32
mechanism. Here, when an ion is transferred from the aqueous phase to the organic phase, another ion
is transferred in the other direction to maintain the charge balance. This additional ion is often a hydrogen ion
; for ion exchange mechanisms, the distribution ratio is often a function of pH
. An example of an ion exchange extraction would be the extraction of americium
by a combination of terpyridine
and a carboxylic acid
in tert-butyl
benzene
. In this case
DAm = k terpyridine
1carboxylic acid
3H+
−3
Another example is the extraction of zinc
, cadmium
, or lead
by a dialkyl phosphinic acid (R2PO2H) into a nonpolar diluent
such as an alkane
. A non-polar diluent favours the formation of uncharged non-polar metal
complexes.
Some extraction systems are able to extract metals by both the solvation and ion exchange mechanisms; an example of such a system is the americium (and lanthanide
) extraction from nitric acid
by a combination of 6,6'-bis-(5,6-dipentyl
-1,2,4-triazin-3-yl)-2,2'-bipyridine
and 2-bromohexanoic acid
in tert-butyl
benzene
. At both high- and low-nitric acid concentrations, the metal distribution ratio is higher than it is for an intermediate nitric acid concentration.
concentration is high, it is possible to extract americium
as an anionic nitrate complex if the mixture contains a lipophilic
quaternary ammonium salt.
An example that is more likely to be encountered by the 'average' chemist is the use of a phase transfer catalyst
. This is a charged species that transfers another ion
to the organic phase. The ion reacts and then forms another ion, which is then transferred back to the aqueous phase.
For instance, the 31.1 kJ mol
−1 is required to transfer an acetate
anion into nitrobenzene, while the energy required to transfer a chloride anion from an aqueous phase to nitrobenzene is 43.8 kJ mol−1. Hence, if the aqueous phase in a reaction is a solution of sodium acetate
while the organic phase is a nitrobenzene solution of benzyl chloride
, then, when a phase transfer catalyst, the acetate anions can be transferred from the aqueous layer where they react with the benzyl
chloride
to form benzyl acetate and a chloride anion. The chloride anion is then transferred to the aqueous phase. The transfer energies of the anions contribute to that given out by the reaction.
A 43.8 to 31.1 kJ mol−1 = 12.7 kJ mol−1 of additional energy is given out by the reaction when compared with energy if the reaction had been done in nitrobenzene
using one equivalent weight
of a tetraalkylammonium acetate.
or nickel
can be very slow because the rate of ligand exchange at these metal centers is much lower than the rates for iron
or silver
complexes.
then the presence of iodide
in the aqueous phase can alter the extraction chemistry.
Instead of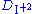 being a constant it becomes
being a constant it becomes 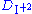 = kI2.Organic/[I2.Aqueous] I-.Aqueous
= kI2.Organic/[I2.Aqueous] I-.Aqueous
This is because the iodine
reacts with the iodide
to form I3-. The I3- anion is an example of a polyhalide anion that is quite common.
After use, the organic phase may be subjected to a cleaning step to remove any degradation products; for instance, in PUREX plants, the used organic phase is washed with sodium carbonate
solution to remove any dibutyl hydrogen phosphate or butyl dihydrogen phosphate that might be present.
While solvent extraction is often done on a small scale by synthetic lab chemists using a separatory funnel
or Craig apparatus, it is normally done on the industrial scale using machines that bring the two liquid phases into contact with each other. Such machines include centrifugal contactors, thin layer extractors, spray columns, pulsed columns
, and mixer-settlers.
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in two different immiscible
Miscibility
Miscibility is the property of liquids to mix in all proportions, forming a homogeneous solution. In principle, the term applies also to other phases , but the main focus is usually on the solubility of one liquid in another...
liquids, usually water and an organic solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
. It is an extraction of a substance from one liquid phase into another liquid phase. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a separatory funnel
Separatory funnel
A separatory funnel, also known as separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate the components of a mixture between two immiscible solvent phases of different densities Typically, one of the...
. This type of process is commonly performed after a chemical reaction as part of the work-up.
The term partitioning is commonly used to refer to the underlying chemical and physical processes involved in liquid–liquid extraction but may be fully synonymous. The term solvent extraction can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.
Solvent extraction is used in nuclear reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
, ore processing, the production of fine organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s, the processing of perfume
Perfume
Perfume is a mixture of fragrant essential oils and/or aroma compounds, fixatives, and solvents used to give the human body, animals, objects, and living spaces "a pleasant scent"...
s, the production of vegetable oils
Vegetable fats and oils
Vegetable fats and oils are lipid materials derived from plants. Physically, oils are liquid at room temperature, and fats are solid. Chemically, both fats and oils are composed of triglycerides, as contrasted with waxes which lack glycerin in their structure...
and biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
, and other industries.
Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salt
Molten salt
Molten salt refers to a salt that is in the liquid phase that is normally a solid at standard temperature and pressure . A salt which is normally liquid at STP is usually called a room temperature ionic liquid, although technically molten salts are a class of ionic liquids.-Uses:Molten salts have...
s, metals can be extracted from one phase to the other. This is related to a mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
where a metal can be reduced, the metal will often then dissolve in the mercury to form an amalgam
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
that modifies its electrochemistry greatly. For example, it is possible for sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
cations to be reduced at a mercury cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
to form sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
or fine solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
can be used to stabilize an emulsion
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible . Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion is used when both the dispersed and the...
, or third phase
Third phase
Third phase is the term for a stable emulsion which forms in a solvent extraction system when the original two phases are mixed.The third phase can be caused by a detergent or a fine solid...
.
Distribution ratio
In solvent extraction, a distribution ratio is often quoted as a measure of how well-extracted a species is. The distribution ratio (D) is equal to the concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of a solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
in the organic phase divided by its concentration in the aqueous phase. Depending on the system, the distribution ratio can be a function of temperature, the concentration of chemical species in the system, and a large number of other parameters.
Note that D is related to the ΔG of the extraction process.
Sometimes, the distribution ratio is referred to as the partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
, which is often expressed as the logarithm
Logarithm
The logarithm of a number is the exponent by which another fixed value, the base, has to be raised to produce that number. For example, the logarithm of 1000 to base 10 is 3, because 1000 is 10 to the power 3: More generally, if x = by, then y is the logarithm of x to base b, and is written...
. Note that a distribution ratio for uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
between two inorganic solids (zirconolite
Zirconolite
Zirconolite is a mineral, calcium zirconium titanate; formula CaZrTi2O7. Some examples of the mineral may also contain thorium, uranium, cerium, niobium and iron; the presence of thorium and/or uranium would make the mineral radioactive....
and perovskite
Perovskite
A perovskite structure is any material with the same type of crystal structure as calcium titanium oxide , known as the perovskite structure, or XIIA2+VIB4+X2−3 with the oxygen in the face centers. Perovskites take their name from this compound, which was first discovered in the Ural mountains of...
) has been reported.
In solvent extraction, two immiscible liquids are shaken together. The more polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
solutes dissolve preferentially in the more polar solvent, and the less polar solutes in the less polar solvent. In this experiment, the nonpolar halogens preferentially dissolve in the nonpolar mineral oil.
Separation factors
The separation factor is one distribution ratio divided by another; it is a measure of the ability of the system to separate two solutes. For instance, if the distribution ratio for nickelNickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
(DNi) is 10 and the distribution ratio for silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
(DAg) is 100, then the silver/nickel separation factor (SFAg/Ni) is equal to DAg/DNi = SFAg/Ni = 10.
Decontamination factor
This is used to express the ability of a process to remove a contaminant from a product. For instance, if a process is fed with a mixture of 1:9 cadmiumCadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
to indium
Indium
Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
, and the product is a 1:99 mixture of cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
and indium
Indium
Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
, then the decontamination factor (for the removal of cadmium) of the process is 0.1 / 0.01 = 10.
Slopes of graphs
The easy way to work out the extraction mechanism is to draw graphs and measure the slopes. If for an extraction system the D value is proportional to the square of the concentration of a reagent (Z) then the slope of the graph of log10(D) against log10(Batchwise single stage extractions
This is commonly used on the small scale in chemical labs. It is normal to use a separating funnel. For instance, if a chemist were to extract anisoleAnisole
Anisole, or methoxybenzene, is the organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances...
from a mixture
Mixture
In chemistry, a mixture is a material system made up by two or more different substances which are mixed together but are not combined chemically...
of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and 5% acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
using ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
, then the anisole will enter the organic phase. The two phases would then be separated.
The acetic acid can then be scrubbed (removed) from the organic phase by shaking the organic extract with sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
. The acetic acid reacts with the sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
to form sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
.
Multistage countercurrent continuous processes
These are commonly used in industryIndustry
Industry refers to the production of an economic good or service within an economy.-Industrial sectors:There are four key industrial economic sectors: the primary sector, largely raw material extraction industries such as mining and farming; the secondary sector, involving refining, construction,...
for the processing of metals such as the lanthanides; because the separation factors between the lanthanides are so small many extraction stages are needed. In the multistage processes, the aqueous raffinate
Raffinate
Raffinating something is a technique used in metallurgy to remove impurities from liquid material. There are many different kinds of raffination, for example you can use vacuum to extract hydrogen from metals....
from one extraction unit is fed to the next unit as the aqueous feed, while the organic phase is moved in the opposite direction. Hence, in this way, even if the separation between two metals in each stage is small, the overall system can have a higher decontamination factor.
Multistage countercurrent
Countercurrent exchange
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or...
arrays have been used for the separation of lanthanides. For the design of a good process, the distribution ratio should be not too high (>100) or too low (<0.1) in the extraction portion of the process. It is often the case that the process will have a section for scrubbing unwanted metals from the organic phase, and finally a stripping
Stripping (chemistry)
Stripping is a physical separation process where one or more components are removed from a liquid stream by a vapor stream. In industrial applications the liquid and vapor streams can have co-current or countercurrent flows. Stripping is usually carried out in either a packed or trayed column.-...
section to obtain the metal back from the organic phase.
Multistage Podbielniak contactor centrifuges produce three to five stages of theoretical extraction in a single countercurrent pass, and are used in fermentation-based pharmaceutical and food additive production facilities.
Extraction without chemical change
Some solutes such as noble gasNoble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es can be extracted from one phase to another without the need for a chemical reaction (see absorption). This is the simplest type of solvent extraction. When a solvent is extracted, two immiscible liquids are shaken together. The more polar solutes dissolve preferentially in the more polar solvent, and the less polar solutes in the less polar solvent. Some solutes that do not at first sight appear to undergo a reaction during the extraction process do not have distribution ratio that is independent of concentration. A classic example is the extraction of carboxylic acids (HA) into nonpolar media such as benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. Here, it is often the case that the carboxylic acid will form a dimer in the organic layer so the distribution ratio will change as a function of the acid concentration (measured in either phase).
For this case, the extraction constant k is described by k = HAorganic2/HAaqueous
Solvation mechanism
Using solvent extraction it is possible to extract uraniumUranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
, or thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
from acid solutions. One solvent used for this purpose is the organophosphate
Organophosphate
An organophosphate is the general name for esters of phosphoric acid. Phosphates are probably the most pervasive organophosphorus compounds. Many of the most important biochemicals are organophosphates, including DNA and RNA as well as many cofactors that are essential for life...
tri-n-butyl phosphate. The PUREX
PUREX
PUREX is an acronym standing for Plutonium - URanium EXtraction — de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid-liquid extraction ion-exchange.The PUREX process was invented by Herbert H. Anderson and...
process that is commonly used in nuclear reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
uses a mixture of tri-n-butyl phosphate and an inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
(kerosene
Kerosene
Kerosene, sometimes spelled kerosine in scientific and industrial usage, also known as paraffin or paraffin oil in the United Kingdom, Hong Kong, Ireland and South Africa, is a combustible hydrocarbon liquid. The name is derived from Greek keros...
), the uranium(VI) are extracted from strong nitric acid and are back-extracted (stripped) using weak nitric acid. An organic soluble uranium complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
[UO2(TBP)2(NO3)2] is formed, then the organic layer bearing the uranium is brought into contact with a dilute
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
nitric acid solution; the equilibrium is shifted away from the organic soluble uranium complex and towards the free TBP and uranyl nitrate
Uranyl nitrate
Uranyl nitrate is a water soluble yellow uranium salt. The yellow-green crystals of uranium nitrate hexahydrate are triboluminescent.Uranyl nitrate can be prepared by reaction of uranium salts with nitric acid...
in dilute nitric acid. The plutonium(IV) forms a similar complex to the uranium(VI), but it is possible to strip the plutonium in more than one way; a reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
that converts the plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
to the trivalent oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
can be added. This oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
does not form a stable complex with TBP and nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
unless the nitrate concentration is very high (circa 10 mol/L nitrate is required in the aqueous phase). Another method is to simply use dilute nitric acid as a stripping agent for the plutonium. This PUREX chemistry is a classic example of a solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
extraction.
Here in this case DU = k TBP
Tributyl phosphate
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the formula 3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of orthophosphoric acid with n-butanol.- Production :Tributyl phosphate is manufactured by...
2NO32
Ion exchange mechanism
Another extraction mechanism is known as the ion exchangeIon exchange
Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion...
mechanism. Here, when an ion is transferred from the aqueous phase to the organic phase, another ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
is transferred in the other direction to maintain the charge balance. This additional ion is often a hydrogen ion
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
; for ion exchange mechanisms, the distribution ratio is often a function of pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
. An example of an ion exchange extraction would be the extraction of americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
by a combination of terpyridine
Terpyridine
Terpyridine is a heterocyclic compound derived from pyridine. This colourless solid is used as a ligand in coordination chemistry.-Synthesis:...
and a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
in tert-butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. In this case
DAm = k terpyridine
Terpyridine
Terpyridine is a heterocyclic compound derived from pyridine. This colourless solid is used as a ligand in coordination chemistry.-Synthesis:...
1carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
3H+
Hydron (chemistry)
In chemistry, a hydron is the general name for a cationic form of atomic hydrogen : most commonly a "proton". However, hydron includes cations of hydrogen regardless of their isotopic composition: thus it refers collectively to protons , deuterons , and tritons...
−3
Another example is the extraction of zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
, or lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
by a dialkyl phosphinic acid (R2PO2H) into a nonpolar diluent
Diluent
A diluent is a diluting agent.Certain fluids are too viscous to be pumped easily or too dense to flow from one particular point to the other. This can be problematic, because it might not be economically feasible to transport such fluids in this state.To ease this restricted movement, diluents...
such as an alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
. A non-polar diluent favours the formation of uncharged non-polar metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
complexes.
Some extraction systems are able to extract metals by both the solvation and ion exchange mechanisms; an example of such a system is the americium (and lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
) extraction from nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
by a combination of 6,6'-bis-(5,6-dipentyl
Pentyl
In organic chemistry, pentyl is a five-carbon alkyl substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane. In older literature, the common non-systematic name "amyl" was often used for the pentyl group....
-1,2,4-triazin-3-yl)-2,2'-bipyridine
2,2'-Bipyridine
2,2'-Bipyridine is a organic compound with the formula . This colorless solid, commonly abbreviated bipy or bpy , is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals...
and 2-bromohexanoic acid
Hexanoic acid
Hexanoic acid , is the carboxylic acid derived from hexane with the general formula C5H11COOH. It is a colorless oily liquid with an odor that is fatty, cheesy, waxy, and like that of goats or other barnyard animals...
in tert-butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. At both high- and low-nitric acid concentrations, the metal distribution ratio is higher than it is for an intermediate nitric acid concentration.
Ion pair extraction
It is possible by careful choice of counterion to extract a metal. For instance, if the nitrateNitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
concentration is high, it is possible to extract americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
as an anionic nitrate complex if the mixture contains a lipophilic
Lipophilic
Lipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
quaternary ammonium salt.
An example that is more likely to be encountered by the 'average' chemist is the use of a phase transfer catalyst
Phase transfer catalyst
In chemistry, a phase transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. Phase transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but...
. This is a charged species that transfers another ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
to the organic phase. The ion reacts and then forms another ion, which is then transferred back to the aqueous phase.
For instance, the 31.1 kJ mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
−1 is required to transfer an acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
anion into nitrobenzene, while the energy required to transfer a chloride anion from an aqueous phase to nitrobenzene is 43.8 kJ mol−1. Hence, if the aqueous phase in a reaction is a solution of sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
while the organic phase is a nitrobenzene solution of benzyl chloride
Benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
, then, when a phase transfer catalyst, the acetate anions can be transferred from the aqueous layer where they react with the benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
to form benzyl acetate and a chloride anion. The chloride anion is then transferred to the aqueous phase. The transfer energies of the anions contribute to that given out by the reaction.
A 43.8 to 31.1 kJ mol−1 = 12.7 kJ mol−1 of additional energy is given out by the reaction when compared with energy if the reaction had been done in nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
using one equivalent weight
Equivalent weight
Equivalent weight is a term which has been used in several contexts in chemistry. In its most general usage, it is the mass of one equivalent, that is the mass of a given substance which will:...
of a tetraalkylammonium acetate.
Aqueous two-phase extraction
Using an aqueous two-phase system, it is possible to generate two immiscible water phases. This can then be used to extract proteins, which would denature if exposed to organic solvents.Kinetics of extraction
It is important to investigate the rate at which the solute is transferred between the two phases, in some cases by an alteration of the contact time it is possible to alter the selectivity of the extraction. For instance, the extraction of palladiumPalladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
or nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
can be very slow because the rate of ligand exchange at these metal centers is much lower than the rates for iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
or silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
complexes.
Aqueous complexing agents
If a complexing agent is present in the aqueous phase then it can lower the distribution ratio. For instance, in the case of iodine being distributed between water and an inert organic solvent such as carbon tetrachlorideCarbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
then the presence of iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
in the aqueous phase can alter the extraction chemistry.
Instead of
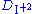 being a constant it becomes
being a constant it becomes 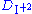 = kI2.Organic/[I2.Aqueous] I-.Aqueous
= kI2.Organic/[I2.Aqueous] I-.AqueousThis is because the iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
reacts with the iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
to form I3-. The I3- anion is an example of a polyhalide anion that is quite common.
Industrial process design
In a typical scenario, an industrial process will use an extraction step in which solutes are transferred from the aqueous phase to the organic phase; this is often followed by a scrubbing stage in which unwanted solutes are removed from the organic phase, then a stripping stage in which the wanted solutes are removed from the organic phase. The organic phase may then be treated to make it ready for use again.After use, the organic phase may be subjected to a cleaning step to remove any degradation products; for instance, in PUREX plants, the used organic phase is washed with sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
solution to remove any dibutyl hydrogen phosphate or butyl dihydrogen phosphate that might be present.
Equipment
Two layers separating during a liquid–liquid extraction.While solvent extraction is often done on a small scale by synthetic lab chemists using a separatory funnel
Separatory funnel
A separatory funnel, also known as separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate the components of a mixture between two immiscible solvent phases of different densities Typically, one of the...
or Craig apparatus, it is normally done on the industrial scale using machines that bring the two liquid phases into contact with each other. Such machines include centrifugal contactors, thin layer extractors, spray columns, pulsed columns
Pulsed columns
Pulsed columns are a type of liquid-liquid extraction equipment; examples of this class of extraction equipment is used at the BNFL plant THORP....
, and mixer-settlers.
Extraction of metals
The extraction methods for a range of metals include:- Cobalt – The extraction of cobalt from hydrochloric acidHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
using alamine 336 in metaMetaMeta- , is a prefix used in English to indicate a concept which is an abstraction from another concept, used to complete or add to the latter....
-xyleneXyleneXylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
. Cobalt can be extracted also using Cyanex 272 {bis-(2,4,4-trimethylpentyl) phosphinic acid}.
- Copper – Copper can be extracted using hydroxyoximeOximeAn oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s as extractants, a recent paper describes an extractant that has a good selectivity for copper over cobaltCobaltCobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
and nickelNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
.
- Neodymium – This rare earth is extracted by di(2-ethyl-hexyl)phosphoric acid into hexaneHexaneHexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
by an ion exchange mechanism.
- Nickel – Nickel can be extracted using di(2-ethyl-hexyl)phosphoric acid and tributyl phosphateTributyl phosphateTributyl phosphate, known commonly as TBP, is an organophosphorus compound with the formula 3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of orthophosphoric acid with n-butanol.- Production :Tributyl phosphate is manufactured by...
in a hydrocarbon diluent (Shellsol).
- Palladium and platinum – Dialkyl sulfides, tributyl phosphate and alkyl amines have been used for extracting these metals.
- Zinc and cadmium – The zinc and cadmium are both extracted by an ion exchange process, the N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) acts as a masking agent for the zinc and an extractant for the cadmium. In the modified Zincex process, zinc is separated from most divalent ions by solvent extraction. D2EHPA (Di (2) ethyl hexyl phosphoric acid) is used for this. A zinc ion replaces the proton from two D2EHPA molecules. To strip the zinc from the D2EHPA, sulfuric acid is used, at a concentration of above 170g/l (typically 240-265g/l).
See also
- centrifugal extractorCentrifugal extractorA centrifugal extractor uses the rotation of the rotor inside a centrifuge to mix two immiscible liquids outside the rotor and to separate the liquids in the field of gravity inside the rotor...
- Acid-base extractionAcid-base extractionAcid-base extraction is a procedure using sequential liquid–liquid extractions to purify acids and bases from mixtures based on their chemical properties....
- Ionic transferIonic transferIonic transfer is a term which can be used to describe the transfer of ions from one liquid phase to another.This is related to the phase transfer catalysts which are a special type of liquid-liquid extraction which is used in synthetic chemistry....
- Multiphasic liquidMultiphasic liquidA multiphasic liquid is a mixture consisting of more than two immiscible liquid phases. Biphasic mixtures consisting of two immiscible phases are very common and usually consist of an organic solvent and an aqueous phase...
- Separatory funnelSeparatory funnelA separatory funnel, also known as separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate the components of a mixture between two immiscible solvent phases of different densities Typically, one of the...
- Work-up (chemistry)
- ITIESITIESITIES is an acronym used in electrochemistry for the "Interface between Two Immiscible Electrolyte Solutions". Usually, one electrolyte is an aqueous electrolyte composed of hydrophilic ions such as NaCl dissolved in water and the other electrolyte is a lipophilic salt such as tetrabutylammonium...

