
Lattice constant
Encyclopedia
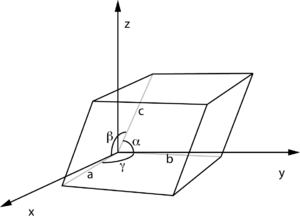
For example the lattice constant for a common carbon diamond is a = 3.57Å at 300 K. The structure is equilateral although its actual shape can not be determined from only the lattice constant. Furthermore, in real applications, typically the average lattice constant is given. As lattice constants have the dimension of length, their SI unit is the meter. Lattice constants are typically on the order of several angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s (i.e. tenths of a nanometre
Nanometre
A nanometre is a unit of length in the metric system, equal to one billionth of a metre. The name combines the SI prefix nano- with the parent unit name metre .The nanometre is often used to express dimensions on the atomic scale: the diameter...
). Lattice constants can be determined using techniques such as X-ray diffraction or with an atomic force microscope
Atomic force microscope
Atomic force microscopy or scanning force microscopy is a very high-resolution type of scanning probe microscopy, with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit...
.
In epitaxial growth
Epitaxy
Epitaxy refers to the deposition of a crystalline overlayer on a crystalline substrate, where the overlayer is in registry with the substrate. In other words, there must be one or more preferred orientations of the overlayer with respect to the substrate for this to be termed epitaxial growth. The...
, the lattice constant is a measure of the structural compatibility between different materials.
Lattice constant matching is important for the growth of thin layer
Thin layer chromatography
Thin layer chromatography is a chromatography technique used to separate mixtures. Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide, or cellulose...
s of materials on other materials; when the constants differ, strains are introduced into the layer, which prevents epitaxial growth
Epitaxy
Epitaxy refers to the deposition of a crystalline overlayer on a crystalline substrate, where the overlayer is in registry with the substrate. In other words, there must be one or more preferred orientations of the overlayer with respect to the substrate for this to be termed epitaxial growth. The...
of thicker layers without defects.
Lattice matching
Matching of lattice structures between two different semiconductor materials allows a region of band gapBand gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
change to be formed in a material without introducing a change in crystal structure. This allows construction of advanced light-emitting diode
Light-emitting diode
A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
s and diode lasers.
For example, gallium arsenide, aluminium gallium arsenide
Aluminium gallium arsenide
Aluminium gallium arsenide is a semiconductor material with very nearly the same lattice constant as GaAs, but a larger bandgap. The x in the formula above is a number between 0 and 1 - this indicates an arbitrary alloy between GaAs and AlAs.The bandgap varies between 1.42 eV and 2.16 eV...
, and aluminium arsenide
Aluminium arsenide
Aluminium arsenide or aluminum arsenide is a semiconductor material with almost the same lattice constant as gallium arsenide and aluminium gallium arsenide and wider band gap than gallium arsenide.-Properties:...
have almost equal lattice constants, making it possible to grow almost arbitrarily thick layers of one on the other one.
Lattice grading
Typically, films of different materials grown on the previous film or substrate are chosen to match the lattice constant of the prior layer to minimize film stress.An alternative method is to grade the lattice constant from one value to another by a controlled altering of the alloy ratio during film growth. The beginning of the grading layer will have a ratio to match the underlying lattice and the alloy at the end of the layer growth will match the desired final lattice for the following layer to be deposited.
The rate of change in the alloy must be determined by weighing the penalty of layer strain, and hence defect density, against the cost of the time in the epitaxy tool.
For example, indium gallium phosphide
Indium gallium phosphide
Indium gallium phosphide , also called gallium indium phosphide , is a semiconductor composed of indium, gallium and phosphorus...
layers with a band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
above 1.9 eV can be grown on gallium arsenide wafers with index grading.

