Ketene
Encyclopedia
A ketene is an organic compound
of the form R'R
, the simplest ketene, where R' and R
atoms.
Ketenes were first studied as a class by Hermann Staudinger
.
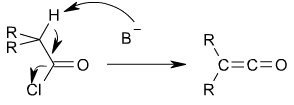
s by an elimination reaction
in which HCl
is lost:
In this reaction, a base, usually triethylamine
, removes the acid
ic proton
alpha to the carbonyl
group, inducing the formation of the carbon-carbon double bond and the loss of a chloride
ion.
Ketenes can also be formed from α-diazoketones by Wolff rearrangement
.
Phenylacetic acid
in the presence of a base will lose water to produce phenylketene due to the high acidity of the alpha proton.
Ketenes can be formed by pyrolysis
(thermal cracking) of acetone
:
This reaction is called the Schmidlin ketene synthesis.
s. They will also undergo [2+2] cycloaddition reactions with electron-rich alkyne
s to form cyclobutenones, or carbonyl groups to form beta-lactone
s. With imine
s beta-lactam
s are formed. This is the Staudinger synthesis
, a facile route to this important class of compounds.
Reactions between diol
s (HO-R-OH) and bis-ketenes (O=C=CH-R'-CH=C=O) yield polyester
s with a repeat unit of (-O-R-O-CO-R'-CO-).
Ethyl acetoacetate
, a very important starting material in organic synthesis, can be prepared using a diketene in reaction with ethanol
. They directly form ethyl acetoacetate, and the yield is good, so this method is used industrially.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
of the form R'R
Ethenone
Ethenone is the formal name for ketene, an organic compound with formula C2H2O or H2C=C=O. It is the simplest member of the ketene class. It is a tautomer of ethynol.- Properties :...
, the simplest ketene, where R' and R
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atoms.
Ketenes were first studied as a class by Hermann Staudinger
Hermann Staudinger
- External links :* Staudinger's * Staudinger's Nobel Lecture *....
.
Formation
Ketenes can be prepared from acyl chlorideAcyl chloride
In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,...
s by an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
in which HCl
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
is lost:
In this reaction, a base, usually triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
, removes the acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
alpha to the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group, inducing the formation of the carbon-carbon double bond and the loss of a chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
ion.
Ketenes can also be formed from α-diazoketones by Wolff rearrangement
Wolff rearrangement
The Wolff rearrangement is a rearrangement reaction converting a α-diazo-ketone into a ketene. This reaction was first reported by Ludwig Wolff in 1912....
.
Phenylacetic acid
Phenylacetic acid
Phenylacetic acid is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a disagreeable odor...
in the presence of a base will lose water to produce phenylketene due to the high acidity of the alpha proton.
Ketenes can be formed by pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
(thermal cracking) of acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
:
- CH3−CO−CH3 + ΔT → CH2=C=O + CH4
This reaction is called the Schmidlin ketene synthesis.
Reactions
Ketenes are generally very reactive, and participate in various cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s. They will also undergo [2+2] cycloaddition reactions with electron-rich alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s to form cyclobutenones, or carbonyl groups to form beta-lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s. With imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s beta-lactam
Beta-lactam
A β-lactam ring, is a four-membered lactam. It is named as such, because the nitrogen atom is attached to the β-carbon relative to the carbonyl...
s are formed. This is the Staudinger synthesis
Staudinger synthesis
The Staudinger synthesis, named after the German chemist Hermann Staudinger, is a method to prepare β-lactams. An imine reacts with a ketene in a formal [2+2]-cycloaddition to give the cyclic amide.-History:...
, a facile route to this important class of compounds.
Reactions between diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s (HO-R-OH) and bis-ketenes (O=C=CH-R'-CH=C=O) yield polyester
Polyester
Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate...
s with a repeat unit of (-O-R-O-CO-R'-CO-).
Ethyl acetoacetate
Ethyl acetoacetate
The organic compound ethyl acetoacetate is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B1; as well as...
, a very important starting material in organic synthesis, can be prepared using a diketene in reaction with ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
. They directly form ethyl acetoacetate, and the yield is good, so this method is used industrially.