
Kapustinskii equation
Encyclopedia
The Kapustinskii equation calculates the Lattice Energy
UL for an ionic crystal
, which is experimentally difficult to determine. It is named after Anatoli Fedorovich Kapustinskii
who published the formula in 1956.
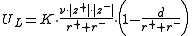
The calculated lattice energy gives a good estimation; the real value differs in most cases by less than 5 %.
Furthermore, one is able to determine the ionic radii
using the Kapustinskii equation when the lattice energy is known. This is useful for rather complex ions like sulfate
(SO) or phosphate
(PO).
Lattice energy
The lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound. It is usually defined as the enthalpy of formation of the ionic compound from gaseous ions and as such is invariably exothermic. Lattice energy may also be defined as the energy required to completely...
UL for an ionic crystal
Ionic crystal
An ionic crystal is a crystal consisting of ions bound together by their electrostatic attraction. Examples of such crystals are the alkali halides, including potassium fluoride, potassium chloride, potassium bromide, potassium iodide, sodium fluoride, and other combinations of sodium, caesium,...
, which is experimentally difficult to determine. It is named after Anatoli Fedorovich Kapustinskii
Anatoli Fedorovich Kapustinskii
Anatoli Fedorovich Kapustinskii was a Polish/Russian chemist. He derived the Kapustinskii equation that allows an estimation of the lattice energy of an ionic crystal.-Biography:...
who published the formula in 1956.
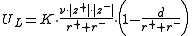
| where | K = 1.2025 J·m·mol−1 |
| d = 3.45 m | |
| ν is the number of ion Ion An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a... s in the empirical formula, |
|
| z+ and z− are the numbers of elementary charge on the cation and anion, respectively, and | |
| r+ and r− are the radii of the cation and anion, respectively. |
The calculated lattice energy gives a good estimation; the real value differs in most cases by less than 5 %.
Furthermore, one is able to determine the ionic radii
Ionic radius
Ionic radius, rion, is the radius of an atom's ion. Although neither atoms nor ions have sharp boundaries, it is important to treat them as if they are hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice...
using the Kapustinskii equation when the lattice energy is known. This is useful for rather complex ions like sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
(SO) or phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
(PO).
Literature
- A. F. Kapustinskii; Z. Phys. Chem. Abt. B Nr. 22, 1933, pp. 257 ff.
- A. F. Kapustinskii; Zhur. Fiz. Khim. Nr. 5, 1943, pp. 59 ff.
- A. F. Kapustinskii: Lattice energy of ionic crystals. In: Quart. Rev. Chem. Soc. Nr. 10, 1956, pp. 283–294.

