K-Casein
Encyclopedia
K-Casein is a mammalian milk
protein
involved in a number of important physiological processes. In the gut
, the ingested protein is split into an insoluble peptide
(para kappa-casein) and a soluble hydrophilic glycopeptide (caseinomacropeptide). Caseinomacropeptide is responsible for increased efficiency of digestion, prevention of neonate hypersensitivity to ingested proteins, and inhibition of gastric pathogens.http://www.ebi.ac.uk/interpro/DisplayIproEntry?ac=IPR000117
 Caseins are a family of phosphoproteins (αS1, αS2, β, κ) that account for nearly 80% of bovine milk proteins (Lucey et al., 2003) and that form soluble aggregates because of the κ-casein molecules that stabilize the micellar structure. There are several models that account for the special conformation of casein in the micelles (Dalgleish, 1998). One of them proposes that the micellar nucleus is formed by several submicelles, the periphery consisting of microvellosities of κ-casein (Walstra, 1979; Lucey, 2002). Another model suggests that the nucleus is formed by casein-interlinked fibrils (Holt, 1992). Finally, the most recent model (Horne, 1998) proposes a double link among the caseins for gelling to take place. All 3 models consider micelles as colloidal particles formed by casein aggregates wrapped up in soluble κ-casein molecules.
Caseins are a family of phosphoproteins (αS1, αS2, β, κ) that account for nearly 80% of bovine milk proteins (Lucey et al., 2003) and that form soluble aggregates because of the κ-casein molecules that stabilize the micellar structure. There are several models that account for the special conformation of casein in the micelles (Dalgleish, 1998). One of them proposes that the micellar nucleus is formed by several submicelles, the periphery consisting of microvellosities of κ-casein (Walstra, 1979; Lucey, 2002). Another model suggests that the nucleus is formed by casein-interlinked fibrils (Holt, 1992). Finally, the most recent model (Horne, 1998) proposes a double link among the caseins for gelling to take place. All 3 models consider micelles as colloidal particles formed by casein aggregates wrapped up in soluble κ-casein molecules.
Milk-clotting proteases act on the soluble portion, κ-casein, thus originating an unstable micellar state that
results in clot formation (Vasbinder et al.., 2003).
 Chymosin
Chymosin
(EC 3.4.23.4) is an aspartic protease that specifically hydrolyzes the peptide bond in Phe105-Met106 of κ- casein and is considered to be the most efficient protease for the cheesemaking industry (Rao et al.., 1998). However, there are milk-clotting proteases able to cleave other peptide bonds in the κ-casein chain, such as the endothiapepsin produced by Endothia parasitica (Drohse et al.., 1989). There are also several milk-clotting proteases that, being able to cleave the Phe105-Met106 bond in the κ-casein molecule, also cleave other peptide bonds in other caseins, such as those produced by Cynara cardunculus (Lucey, 2002; Esteves et al.., 2003; Silva and Malcata, 2005) or even bovine chymosin (Kobayashi, 2004). This allows the manufacture of different cheeses with a variety of rheological and organoleptic properties.
The milk-clotting process consists of 3 main phases (Carlson et al.., 1987a):
Each step follows a different kinetic
pattern, the limiting step in milk-clotting being the degradation rate
of κ-casein. The kinetic pattern of the second step of the milk-clotting process is influenced by the cooperative nature of micellar flocculation (Carlson et al.., 1987b; Silva and Malcata, 2005) whereas the rheological properties of the gel formed depend on the type of action of the proteases, the type of milk, and the patterns of casein proteolysis (Silva and Malcata, 2005). The overall process is influenced by several different factors, such as pH or temperature (Esteves et al.., 2003; Vasbinder et al.., 2003).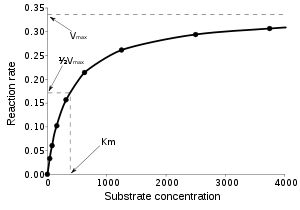 The conventional way of quantifying a given milkclotting enzyme (Poza et al.., 2003) employs milk as the substrate and determines the time elapsed before the appearance of milk clots. However, milk clotting may take place without the participation of enzymes because of variations in physicochemical factors, such as low pH or high temperature (Lucey, 2002; Lucey et al.., 2003; Vasbinder et al.., 2003). Consequently, this may lead to confusing and irreproducible results, particularly when the enzymes have low activity. At the same time, the classical method is not specific enough, in terms of setting the precise onset of milk gelation, such that the determination of the enzymatic units involved becomes difficult and unclear. Furthermore, although it has been reported that κ-casein hydrolysis follows typical Michaelis-Menten kinetics (Carlson et al.., 1987a), it is difficult to determine with the classic milk-clotting assay.
The conventional way of quantifying a given milkclotting enzyme (Poza et al.., 2003) employs milk as the substrate and determines the time elapsed before the appearance of milk clots. However, milk clotting may take place without the participation of enzymes because of variations in physicochemical factors, such as low pH or high temperature (Lucey, 2002; Lucey et al.., 2003; Vasbinder et al.., 2003). Consequently, this may lead to confusing and irreproducible results, particularly when the enzymes have low activity. At the same time, the classical method is not specific enough, in terms of setting the precise onset of milk gelation, such that the determination of the enzymatic units involved becomes difficult and unclear. Furthermore, although it has been reported that κ-casein hydrolysis follows typical Michaelis-Menten kinetics (Carlson et al.., 1987a), it is difficult to determine with the classic milk-clotting assay.
To overcome this, several alternative methods have been proposed, such as the determination of halo diameter in agar-gelified milk (Poza et al.., 2003), colorimetric measurement (Hull, 1947), or determination of the rate of degradation of casein previously labeled with either a radioactive tracer (Christen, 1987) or a fluorochrome compound (Twining, 1984). All these methods use casein as the substrate to quantify proteolytic or milkclotting activities.
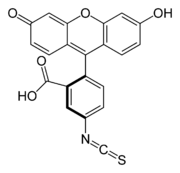 K-casein labeled with the fluorochrome fluorescein isothiocyanate
K-casein labeled with the fluorochrome fluorescein isothiocyanate
(FITC) to yield the fluorescein thiocarbamoyl (FTC) derivative. This substrate is used to deternimate the milk clotting activity of proteases (Ageitos, et al., 2006).
FTC-κ-casein method affords accurate and precise determinations of κ-caseinolytic degradation, the first step in the milk-clotting process. This method is the result of a modification to the one described by S.S. Twining (1984). The main modification was substituting the substrate previously used (casein
) by -casein labeled with the fluorochrome fluorescein isothiocyanate (FITC) to yield the fluorescein thiocarbamoyl (FTC) derivative. This variation allows quantification of the -casein molecules degraded in a more precise and specific way, detecting only those enzymes able to degrade such molecules. The method described by Twining (1984), however, was designed to detect the proteolytic activity of a considerably large variety of enzymes.
FTC-κ-casein allows the detection of different types of proteases at levels when no milk clotting is yet apparent, unveiling its higher sensitivity over currently used assay procedures.
Therefore, the method may find application as an indicator during the purification or characterization of new
milk-clotting enzymes.
Milk
Milk is a white liquid produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to the baby and can reduce the risk of many...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
involved in a number of important physiological processes. In the gut
Gut (zoology)
In zoology, the gut, also known as the alimentary canal or alimentary tract, is a tube by which bilaterian animals transfer food to the digestion organs. In large bilaterians the gut generally also has an exit, the anus, by which the animal disposes of solid wastes...
, the ingested protein is split into an insoluble peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
(para kappa-casein) and a soluble hydrophilic glycopeptide (caseinomacropeptide). Caseinomacropeptide is responsible for increased efficiency of digestion, prevention of neonate hypersensitivity to ingested proteins, and inhibition of gastric pathogens.http://www.ebi.ac.uk/interpro/DisplayIproEntry?ac=IPR000117
Structure


Milk-clotting proteases act on the soluble portion, κ-casein, thus originating an unstable micellar state that
results in clot formation (Vasbinder et al.., 2003).
Milk Clotting

Chymosin
Chymosin or rennin is an enzyme found in rennet. It is produced by cows in the lining of the abomasum...
(EC 3.4.23.4) is an aspartic protease that specifically hydrolyzes the peptide bond in Phe105-Met106 of κ- casein and is considered to be the most efficient protease for the cheesemaking industry (Rao et al.., 1998). However, there are milk-clotting proteases able to cleave other peptide bonds in the κ-casein chain, such as the endothiapepsin produced by Endothia parasitica (Drohse et al.., 1989). There are also several milk-clotting proteases that, being able to cleave the Phe105-Met106 bond in the κ-casein molecule, also cleave other peptide bonds in other caseins, such as those produced by Cynara cardunculus (Lucey, 2002; Esteves et al.., 2003; Silva and Malcata, 2005) or even bovine chymosin (Kobayashi, 2004). This allows the manufacture of different cheeses with a variety of rheological and organoleptic properties.
The milk-clotting process consists of 3 main phases (Carlson et al.., 1987a):
- Enzymatic degradation of κ-casein
- Micellar flocculation
- Gel formation
Each step follows a different kinetic
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
pattern, the limiting step in milk-clotting being the degradation rate
of κ-casein. The kinetic pattern of the second step of the milk-clotting process is influenced by the cooperative nature of micellar flocculation (Carlson et al.., 1987b; Silva and Malcata, 2005) whereas the rheological properties of the gel formed depend on the type of action of the proteases, the type of milk, and the patterns of casein proteolysis (Silva and Malcata, 2005). The overall process is influenced by several different factors, such as pH or temperature (Esteves et al.., 2003; Vasbinder et al.., 2003).

To overcome this, several alternative methods have been proposed, such as the determination of halo diameter in agar-gelified milk (Poza et al.., 2003), colorimetric measurement (Hull, 1947), or determination of the rate of degradation of casein previously labeled with either a radioactive tracer (Christen, 1987) or a fluorochrome compound (Twining, 1984). All these methods use casein as the substrate to quantify proteolytic or milkclotting activities.
FTC-K-Casein Assay

Fluorescein isothiocyanate
Fluorescein isothiocyanate is a derivative of fluorescein used in wide-ranging applications including flow cytometry. FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group , replacing a hydrogen atom on the bottom ring of the structure...
(FITC) to yield the fluorescein thiocarbamoyl (FTC) derivative. This substrate is used to deternimate the milk clotting activity of proteases (Ageitos, et al., 2006).
FTC-κ-casein method affords accurate and precise determinations of κ-caseinolytic degradation, the first step in the milk-clotting process. This method is the result of a modification to the one described by S.S. Twining (1984). The main modification was substituting the substrate previously used (casein
Casein
Casein is the name for a family of related phosphoprotein proteins . These proteins are commonly found in mammalian milk, making up 80% of the proteins in cow milk and between 60% and 65% of the proteins in human milk....
) by -casein labeled with the fluorochrome fluorescein isothiocyanate (FITC) to yield the fluorescein thiocarbamoyl (FTC) derivative. This variation allows quantification of the -casein molecules degraded in a more precise and specific way, detecting only those enzymes able to degrade such molecules. The method described by Twining (1984), however, was designed to detect the proteolytic activity of a considerably large variety of enzymes.
FTC-κ-casein allows the detection of different types of proteases at levels when no milk clotting is yet apparent, unveiling its higher sensitivity over currently used assay procedures.
Therefore, the method may find application as an indicator during the purification or characterization of new
milk-clotting enzymes.

