Japp-Klingemann reaction
Encyclopedia
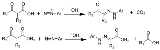
The Japp-Klingemann reaction is a chemical reaction
used to synthesize hydrazone
s from β-keto-acids (or β-keto-esters) and aryl
diazonium salts. The Reaction is named after the chemists Francis Robert Japp
and Felix Klingemann.
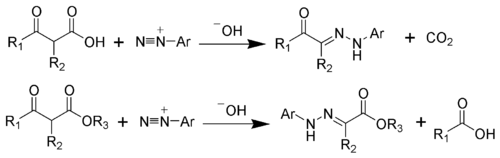 The hydrazone products of the Japp-Klingemann reaction are most often used as intermediates in syntheses
The hydrazone products of the Japp-Klingemann reaction are most often used as intermediates in syntheses
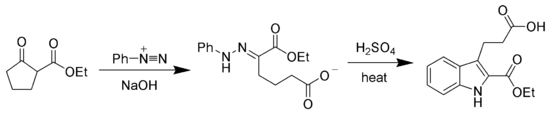
of more complex organic molecules. For example, a phenylhydrazone product can be heated in the presence of strong acid to produce an indole
via the Fischer indole synthesis
.
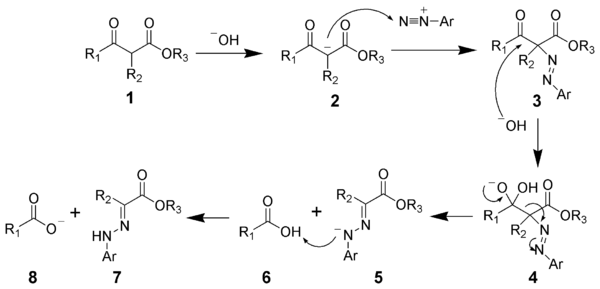
of the β-keto-ester. The nucleophilic addition
of the enolate anion 2 to the diazonium salt produces the azo compound 3. Intermediate 3 has been isolated in rare cases. However, in most cases, the hydrolysis of intermediate 3 produces a tetrahedral intermediate 4, which quickly decomposes to release the carboxylic acid 6. After hydrogen
exchange, the final hydrazone 7 is produced.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
used to synthesize hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
s from β-keto-acids (or β-keto-esters) and aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
diazonium salts. The Reaction is named after the chemists Francis Robert Japp
Francis Robert Japp
Francis Robert Japp was a British chemist who discovered the Japp-Klingemann reaction in 1887.He was born in Dundee, Scotland, the son of James Japp, a minister of the Catholic Apostolic Church. He graduated from St Andrews with an M.A. in 1868 and entered the University of Edinburgh as a student...
and Felix Klingemann.

Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of more complex organic molecules. For example, a phenylhydrazone product can be heated in the presence of strong acid to produce an indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
via the Fischer indole synthesis
Fischer indole synthesis
The Fischer indole synthesis isa chemical reaction that produces the aromatic heterocycle indole from a phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer. Today antimigraine drugs of the triptan class are often...
.

Reaction mechanism
To illustrate the mechanism, the Japp-Klingemann ester variation will be considered. The first step is the deprotonationDeprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the β-keto-ester. The nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of the enolate anion 2 to the diazonium salt produces the azo compound 3. Intermediate 3 has been isolated in rare cases. However, in most cases, the hydrolysis of intermediate 3 produces a tetrahedral intermediate 4, which quickly decomposes to release the carboxylic acid 6. After hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
exchange, the final hydrazone 7 is produced.


