
Isotopes of rutherfordium
Encyclopedia
Rutherfordium
(Rf) is an artificial element, and thus a standard atomic mass
cannot be given. Like all artificial elements, it has no stable isotope
s. The first isotope
to be synthesized was either 259Rf in 1966 or 257Rf in 1969. There are 15 known radioisotopes from 253Rf to 268Rf (2 of which, 266Rf and 268Rf, are unconfirmed) and 4 isomer
s. The longest-lived isotope is 267Rf with an estimated half-life
of 5 hours. The longest directly measured half-life is 263Rf at 11 minutes, and the longest-lived isomer is 261mRf with a half-life of 81 seconds.
208Pb(50Ti,xn)258−xRf (x=1,2,3)
This reaction was first studied in 1974 by the team at Dubna. They measured a spontaneous fission activity assigned to 256Rf. The reaction was further studied in 1985 by the GSI team who measured the decay properties of the isotopes 257Rf and 256Rf. The team were able to determine some initial spectroscopic properties of 257Rf and found that the alpha decay pattern was very complicated.
After an upgrade of their facilities, they repeated the reaction in 1994 with much higher sensitivity and detected some 1100 atoms of 257Rf and 1900 atoms of 256Rf along with 255Rf in the measurement of the 1n,2n and 3n excitation functions. The large amount of decay data for 257Rf allowed the detection of an isomeric level and the construction of a partial decay level structure which confirmed the very complicated alpha decay pattern. They also found evidence for an isomeric level in 255Rf.
The GSI team continued in 2001 with the measurement of the 3n excitation function. In 2002, scientists at the Argonne University in Illinois began their first studies of translawrencium elements with the synthesis and alpha-gamma spectroscopy of 257Rf. In 2004, the GSI began their spectroscopic studies of the 257Rf isotope. In 2007, the Lawrence Berkeley National Laboratory
(LBNL) studied the 2n product, 256Rf, in a search for K-isomers and discovered three such isomers.
 207Pb(50Ti,xn)257−xRf (x=2)
207Pb(50Ti,xn)257−xRf (x=2)
This reaction was first studied in 1974 by the team at Dubna. They measured a spontaneous fission activity assigned to 255Rf. The reaction was further studied in 1985 by the GSI team who measured the decay properties of the isotope 255Rf. A further spectroscopic study was reported in 2000 which led to a first decay level scheme for the isotope. The isomeric level proposed in 1994 was not found. In 2006, the spectroscopy was continued and the decay scheme was confirmed and improved.
206Pb(50Ti,xn)256−xRf (x=1,2)
The team at GSI first studied this reaction in 1994 in an effort to study neutron deficient isotopes of rutherfordium. They were able to detect 255Rf and 144 atoms of the new isotope 254Rf, which decayed by spontaneous fission
.
204Pb(50Ti,xn)254−xRf (x=1)
The team at GSI first studied this reaction in 1994 in an effort to study neutron deficient isotopes of rutherfordium. They were able to detect 14 atoms of the new isotope 253Rf, which decayed by spontaneous fission
.
208Pb(48Ti,xn)256−xRf (x=1)
In 2006, as part of a program looking at the effect of isospin on the mechanism of cold fusion, the team at LBNL studied this reaction. They measured the 1n excitation function and determined that the change of a Ti-50 projectile to a Ti-48 one significantly reduced the yield, in agreement with predictions.
124Sn(136Xe,xn)260-xRf
In an important study, in May 2004, the team at GSI attempted the symmetric synthesis of rutherfordium by attempting to fuse two fission fragments. Theory suggests that there may be an enhancement of the yield. No product atoms were detected and an upper limit of 1000 pb
was estimated for the yield of this reaction.
238U(26Mg,xn)264−xRf (x=3,4,5,6)
The hot fusion reaction using a uranium target was first reported in 2000 by Yuri Lazarev and the team at the Flerov Laboratory of Nuclear Reactions (FLNR). They were able to observe decays from 260Rf and 259Rf in the 4n and 5n channels. They measured yields of 240 pb in the 4n channel and 1.1 nb in the 5n channel.
In 2006, as part of their program on the study of uranium targets in hot fusion reactions, the team at LBNL measured the 4n,5n and 6n excitation functions for this reaction and observed 261Rf in the 3n exit channel.
244Pu(22Ne,xn)266−xRf (x=4,5)
This reaction was reported in 1996 at LBNL in an attempt to study the fission characteristics of 262Rf. The team were able to detect the spontaneous fission
(SF) of 262Rf and determine its half-life as 2.1 s, in contrast to earlier reports of a 47 ms activity. It was suggested that the two half-lives might be related to different isomeric states. The reaction was further studied in 2000 by Yuri Lazarev and the team at Dubna. They were able to observe 69 alpha decays from 261Rf and spontaneous fission
of 262Rf. Later work on hassium
has allowed a reassignment of the 5n product to 261mRf.
242Pu(22Ne,xn)264−xRf (x=3,4?,5?)
The synthesis of element 104 was first attempted in 1964 by the team at Dubna using this reaction. The first study produced evidence for a 0.3 s SF activity tentatively assigned to 260104 or 259104 and an unidentified 8 s SF activity. The former activity was later retracted and the latter activity associated with the now-known 259104. In 1966, in their discovery experiment, the team repeated the reaction using a chemical study of volatile chloride products. The group was able to identify a volatile chloride decaying by short spontaneous fission
with eka-hafnium properties. This gave strong evidence for the formation of [104]Cl4 and the team suggested the name kurchatovium. Although a half-life was not accurately measured, later evidence suggested that the product was most likely 259104. In 1968, the team searched for alpha decay from 260104 but were unable to detect such activity. In 1970, the team repeated the reaction once again and confirmed the ~0.2 s SF activity. They also repeated the chemistry experiment and obtained identical results to their 1966 experiment and calculated a likely half-life of ~0.5 seconds for the SF activity. In 1971, the reaction was repeated again and 0.1 s and 4.5 s SF activities were found. The 4.5 s activity was correctly assigned to 259104. A chemistry experiment in the same year reaffirmed the formation of a 0.3 SF activity for an eka-hafnium product. Later, the 0.1−0.3 s SF activity was retracted as belonging to a kurchatovium isotope but the observation of eka-hafnium reactivity remained and was the basis of their successful claim to discovery. The reaction was further studied in 2000 by Yuri Lazarev at Dubna
. They were able to observe 261Rf in the 3n channel, later reassigned to 261mRf.
242Pu(20Ne,xn)262−xRf
This reaction was first studied in 1964 to assist in the assignments using the analogous reaction with a Ne-22 beam. The Dubna team were unable to detect any 0.3 s spontaneous fission
activities.
The reaction was later studied in 2003 at the Paul Scherrer Institute
(PSI) in Bern, Switzerland. They detected some spontaneous fission
activities but were unable to confirm the formation of 259Rf.
248Cm(22Ne,αxn)266−xRf (x=3?)
This reaction was studied in 1999 at the University of Bern, Switzerland in order to search for the new isotope 263Rf. A rutherfordium fraction was separated and several SF events with long lifetimes and alpha decays with energy 7.8 MeV and 7.9 MeV were observed. A second experiment using a study of the fluoride of rutherfordium products also produced 7.9 MeV alpha decays.
248Cm(18O,xn)266−xRf (x=3?,5)
This reaction was first studied in 1970 by Albert Ghiorso at the LBNL. The team identified 261Rf in the 5n channel using the method of correlation of genetic parent-daughter decays. A half-life of 65 s was determined.
A repeat later that year using cation exchange chromatography indicated that the product did not form a +2 or +3 cation and behaved as eka-hafnium. A study of the properties of rutherfordium isotopes was performed in 1981 at the LBNL. In a series of reactions, a 1.5 s SF activity was identified and assigned to a fermium descendant although later evidence indicates a possible assignment to 262Rf. In contrast, in a subsequent review of isotope properties by Somerville et al. at LBNL in 1985, a 47 ms SF activity was assigned to 262Rf. This assignment has not been verified.
The reaction was further studied in 1991 by Czerwinski et al. at the LBNL. In this experiment, spontaneous fission
activities with long lifetimes were observed in rutherfordium fractions and tentatively assigned to 263Rf. In 1996, chemical studies on the chloride of rutherfordium were published by the LBNL. In this experiment, the half-life was improved to 78 s. A repeat of the experiment in 2000 assessing the volatility of the bromide further refined the half-life to 75 s.
248Cm(16O,xn)264−xRf (x=4)
This reaction was studied in 1969 by Albert Ghiorso at the University of California. The aim was to detect the 0.1–0.3 s SF activity reported at Dubna, assigned to 260104. They were unable to do so, only observing a 10–30 ms SF activity, correctly assigned to 260104. The failure to observe the 0.3 s SF activity identified by Dubna gave the Americans the incentive to name this element rutherfordium.
246Cm(18O,xn)264−xRf
In an attempt to unravel the properties of spontaneous fission
activities in the formation of rutherfordium isotopes, this reaction was performed in 1976 by the FLNR. They observed an 80 ms SF activity. Subsequent work led to the complete retraction of the 0.3s - 0.1s - 80 ms SF activities observed by the Dubna team and associated with background signals.
249Bk(15N,xn)264−xRf (x=4)
This reaction was studied in 1977 by the team in Dubna. They were able to confirm the detection of a 76 ms SF activity. The assignment to rutherfordium isotopes was later retracted. The LBNL re-studied the reaction in 1980 and in 1981 they reported that they were unable to confirm the ~80 ms SF activity. The Dubna team were able to measure a 28 ms SF activity in 1985 and assigned the isotope correctly to 260104.
249Cf(13C,xn)262−xRf (x=4)
This use of californium-249 as a target was first studied by Albert Ghiorso and the team at the University of California in 1969. They were able to observe an 11 ms SF activity which they correctly assigned to 258104.
 249Cf(12C,xn)261−xRf (x=3,4)
249Cf(12C,xn)261−xRf (x=3,4)
In their 1969 discovery experiments, the team at University of California also used carbon-12
beam to irradiate a californium-249 target. They were able to confirm the 11 ms SF activity found with a carbon-13 beam and again correctly assigned to 258104. The actual discovery experiment was the observation of alpha decays genetically linked to 253102 and therefore positively identified as 257104.
In 1973, Bemis and his team at Oak Ridge confirmed the discovery by measuring coincident X-rays from the daughter 253102.
.
with a long half-life
of 10–20 minutes. Alpha particles with energy 7.8-7.9 MeV have also been associated with this nucleus. More recently, a study of hassium
isotope
s allowed the synthesis of an atom of 263Rf decaying by spontaneous fission
with a short half-life
of 8 seconds. These two different decay modes must be associated with two isomeric states. Specific assignments are difficult due to the low number of observed events. It is reasonable to tentatively assign the long life to a meta-stable state, namely 263mRf, and the shorter life to the ground state, namely 263gRf. Further studies are required to allow a definite assignment.
with a half-life
of 78 seconds. Later studies by the GSI
team on the synthesis of copernicium and hassium
isotopes produced conflicting data. In this case, 261Rf was found to undergo 8.52 MeV alpha decay with a short half-life of 4 seconds. Later results indicated a predominant fission branch. These contradictions led to some doubt on the discovery of copernicium. However, it is now understood that the first nucleus belongs to an isomeric meta-stable state, namely 261mRf and the latter to the ground state isomer, namely 261gRf. The discovery and confirmation of 261gRf provided proof for the discovery of copernicium in 1996.


, the isotope 288115 has been observed to decay to 268Db which undergoes spontaneous fission
with a half-life of 29 hours. Given that the electron capture
of 268Db cannot be detected, these SF events may in fact be due to the SF of 268Rf, in which case the half-life of this isotope cannot be extracted.
, the isotope 282113 has been observed to decay to 266Db which undergoes spontaneous fission
with a half-life of 22 minutes. Given that the electron capture
of 266Db cannot be detected, these SF events may in fact be due to the SF of 266Rf, in which case the half-life of this isotope cannot be extracted.
Rutherfordium
Rutherfordium is a chemical element with symbol Rf and atomic number 104, named in honor of New Zealand physicist Ernest Rutherford. It is a synthetic element and radioactive; the most stable known isotope, 267Rf, has a half-life of approximately 1.3 hours.In the periodic table of the elements,...
(Rf) is an artificial element, and thus a standard atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
cannot be given. Like all artificial elements, it has no stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
s. The first isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
to be synthesized was either 259Rf in 1966 or 257Rf in 1969. There are 15 known radioisotopes from 253Rf to 268Rf (2 of which, 266Rf and 268Rf, are unconfirmed) and 4 isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
s. The longest-lived isotope is 267Rf with an estimated half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 5 hours. The longest directly measured half-life is 263Rf at 11 minutes, and the longest-lived isomer is 261mRf with a half-life of 81 seconds.
Table
| nuclide symbol |
Z(p Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... ) |
N(n Neutron The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of... ) |
isotopic mass (u) |
half-life | decay mode(s)Abbreviations: EC: Electron capture Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... SF: Spontaneous fission Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... |
daughter isotope(s) |
nuclear spin |
|---|---|---|---|---|---|---|---|
| excitation energy | |||||||
| 253Rf | 104 | 149 | 253.10069(49)# | 13(5) ms | SF Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... (51%) |
(various) | (7/2)(+#) |
| α Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... (49%) |
249No | ||||||
| 253mRf | 200(150)# keV | 52(14) µs [48(+17-10) µs] |
(1/2)(-#) | ||||
| 254Rf | 104 | 150 | 254.10018(31)# | 23(3) µs | SF (99.7%) | (various) | 0+ |
| α (.3%) | 250No | ||||||
| 255Rf | 104 | 151 | 255.10134(19)# | 1.64(11) s | SF (52%) | (various) | (9/2-)# |
| α (48%) | 251No | ||||||
| 256Rf | 104 | 152 | 256.101166(26) | 6.45(14) ms | SF (96%) | (various) | 0+ |
| α (6%) | 252No | ||||||
| 257Rf | 104 | 153 | 257.10299(11)# | 4.7(3) s | α (79%) | 253No | (1/2+) |
| β+ Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... (18%) |
257Lr | ||||||
| SF (2.4%) | (various) | ||||||
| 257mRf | 114(17) keV | 3.9(4) s | (11/2-) | ||||
| 258Rf | 104 | 154 | 258.10349(22)# | 12(2) ms | SF (87%) | (various) | 0+ |
| α (13%) | 254No | ||||||
| 259Rf | 104 | 155 | 259.10564(8)# | 2.8(4) s | α (93%) | 255No | 7/2+# |
| SF (7%) | (various) | ||||||
| β+ (.3%) | 259Lr | ||||||
| 260Rf | 104 | 156 | 260.10644(22)# | 21(1) ms | SF (98%) | (various) | 0+ |
| α (2%) | 256No | ||||||
| 261Rf | 104 | 157 | 261.10877(3) | 5.5(25) s | α (76%) | 257No | 3/2+# |
| β+ (14%) | 261Lr | ||||||
| SF (10%) | (various) | ||||||
| 261mRf | 70(100)# keV | 81(9) s | β+ | 261Lr | 9/2+# | ||
| α (rare) | 257No | ||||||
| 262Rf | 104 | 158 | 262.10993(30)# | 2.3(4) s | SF (99.2%) | (various) | 0+ |
| α (.8%) | 258No | ||||||
| 262mRf | 600(400)# keV | 47(5) ms | SF | (various) | high | ||
| 263Rf | 104 | 159 | 263.11255(20)# | 11(3) min | SF (70%) | (various) | 3/2+# |
| α (30%) | 259No | ||||||
| 265RfNot directly synthesized, occurs in decay chain Decay chain In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations... of 285Uuq |
104 | 161 | 2.5 min | SF | (various) | ||
| 266RfDiscovery of this isotope is unconfirmedNot directly synthesized, occurs in decay chain of 282Cn | 104 | 162 | 266.11796(58)# | 10# h | 0+ | ||
| 267RfNot directly synthesized, occurs in decay chain of 287Uuq | 104 | 163 | 267.12153(62)# | 5# h [2.3(+980-17) h] |
SF | (various) | |
| 268RfNot directly synthesized, occurs in decay chain of 288Uuq | 104 | 164 | 268.12364(76)# | 1# h | 0+ | ||
History of synthesis of isotopes by cold fusion
This section deals with the synthesis of nuclei of rutherfordium by so-called "cold" fusion reactions. These are processes which create compound nuclei at low excitation energy (~10-20 MeV, hence "cold"), leading to a higher probability of survival from fission. The excited nucleus then decays to the ground state via the emission of one or two neutrons only.208Pb(50Ti,xn)258−xRf (x=1,2,3)
This reaction was first studied in 1974 by the team at Dubna. They measured a spontaneous fission activity assigned to 256Rf. The reaction was further studied in 1985 by the GSI team who measured the decay properties of the isotopes 257Rf and 256Rf. The team were able to determine some initial spectroscopic properties of 257Rf and found that the alpha decay pattern was very complicated.
After an upgrade of their facilities, they repeated the reaction in 1994 with much higher sensitivity and detected some 1100 atoms of 257Rf and 1900 atoms of 256Rf along with 255Rf in the measurement of the 1n,2n and 3n excitation functions. The large amount of decay data for 257Rf allowed the detection of an isomeric level and the construction of a partial decay level structure which confirmed the very complicated alpha decay pattern. They also found evidence for an isomeric level in 255Rf.
The GSI team continued in 2001 with the measurement of the 3n excitation function. In 2002, scientists at the Argonne University in Illinois began their first studies of translawrencium elements with the synthesis and alpha-gamma spectroscopy of 257Rf. In 2004, the GSI began their spectroscopic studies of the 257Rf isotope. In 2007, the Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
The Lawrence Berkeley National Laboratory , is a U.S. Department of Energy national laboratory conducting unclassified scientific research. It is located on the grounds of the University of California, Berkeley, in the Berkeley Hills above the central campus...
(LBNL) studied the 2n product, 256Rf, in a search for K-isomers and discovered three such isomers.

This reaction was first studied in 1974 by the team at Dubna. They measured a spontaneous fission activity assigned to 255Rf. The reaction was further studied in 1985 by the GSI team who measured the decay properties of the isotope 255Rf. A further spectroscopic study was reported in 2000 which led to a first decay level scheme for the isotope. The isomeric level proposed in 1994 was not found. In 2006, the spectroscopy was continued and the decay scheme was confirmed and improved.
206Pb(50Ti,xn)256−xRf (x=1,2)
The team at GSI first studied this reaction in 1994 in an effort to study neutron deficient isotopes of rutherfordium. They were able to detect 255Rf and 144 atoms of the new isotope 254Rf, which decayed by spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
.
204Pb(50Ti,xn)254−xRf (x=1)
The team at GSI first studied this reaction in 1994 in an effort to study neutron deficient isotopes of rutherfordium. They were able to detect 14 atoms of the new isotope 253Rf, which decayed by spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
.
208Pb(48Ti,xn)256−xRf (x=1)
In 2006, as part of a program looking at the effect of isospin on the mechanism of cold fusion, the team at LBNL studied this reaction. They measured the 1n excitation function and determined that the change of a Ti-50 projectile to a Ti-48 one significantly reduced the yield, in agreement with predictions.
124Sn(136Xe,xn)260-xRf
In an important study, in May 2004, the team at GSI attempted the symmetric synthesis of rutherfordium by attempting to fuse two fission fragments. Theory suggests that there may be an enhancement of the yield. No product atoms were detected and an upper limit of 1000 pb
Barn (unit)
A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the...
was estimated for the yield of this reaction.
History of synthesis of isotopes by hot fusion
This section deals with the synthesis of nuclei of rutherfordium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40-50 MeV, hence "hot"), leading to a reduced probability of survival from fission. The excited nucleus then decays to the ground state via the emission of 3-5 neutrons.238U(26Mg,xn)264−xRf (x=3,4,5,6)
The hot fusion reaction using a uranium target was first reported in 2000 by Yuri Lazarev and the team at the Flerov Laboratory of Nuclear Reactions (FLNR). They were able to observe decays from 260Rf and 259Rf in the 4n and 5n channels. They measured yields of 240 pb in the 4n channel and 1.1 nb in the 5n channel.
In 2006, as part of their program on the study of uranium targets in hot fusion reactions, the team at LBNL measured the 4n,5n and 6n excitation functions for this reaction and observed 261Rf in the 3n exit channel.
244Pu(22Ne,xn)266−xRf (x=4,5)
This reaction was reported in 1996 at LBNL in an attempt to study the fission characteristics of 262Rf. The team were able to detect the spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
(SF) of 262Rf and determine its half-life as 2.1 s, in contrast to earlier reports of a 47 ms activity. It was suggested that the two half-lives might be related to different isomeric states. The reaction was further studied in 2000 by Yuri Lazarev and the team at Dubna. They were able to observe 69 alpha decays from 261Rf and spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
of 262Rf. Later work on hassium
Hassium
Hassium is a synthetic element with the symbol Hs and atomic number 108. It is the heaviest member of the group 8 elements. The element was first observed in 1984...
has allowed a reassignment of the 5n product to 261mRf.
242Pu(22Ne,xn)264−xRf (x=3,4?,5?)
The synthesis of element 104 was first attempted in 1964 by the team at Dubna using this reaction. The first study produced evidence for a 0.3 s SF activity tentatively assigned to 260104 or 259104 and an unidentified 8 s SF activity. The former activity was later retracted and the latter activity associated with the now-known 259104. In 1966, in their discovery experiment, the team repeated the reaction using a chemical study of volatile chloride products. The group was able to identify a volatile chloride decaying by short spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
with eka-hafnium properties. This gave strong evidence for the formation of [104]Cl4 and the team suggested the name kurchatovium. Although a half-life was not accurately measured, later evidence suggested that the product was most likely 259104. In 1968, the team searched for alpha decay from 260104 but were unable to detect such activity. In 1970, the team repeated the reaction once again and confirmed the ~0.2 s SF activity. They also repeated the chemistry experiment and obtained identical results to their 1966 experiment and calculated a likely half-life of ~0.5 seconds for the SF activity. In 1971, the reaction was repeated again and 0.1 s and 4.5 s SF activities were found. The 4.5 s activity was correctly assigned to 259104. A chemistry experiment in the same year reaffirmed the formation of a 0.3 SF activity for an eka-hafnium product. Later, the 0.1−0.3 s SF activity was retracted as belonging to a kurchatovium isotope but the observation of eka-hafnium reactivity remained and was the basis of their successful claim to discovery. The reaction was further studied in 2000 by Yuri Lazarev at Dubna
Dubna
Dubna is a town in Moscow Oblast, Russia. It has a status of naukograd , being home to the Joint Institute for Nuclear Research, an international nuclear physics research centre and one of the largest scientific foundations in the country. It is also home to MKB Raduga, a defence aerospace company...
. They were able to observe 261Rf in the 3n channel, later reassigned to 261mRf.
242Pu(20Ne,xn)262−xRf
This reaction was first studied in 1964 to assist in the assignments using the analogous reaction with a Ne-22 beam. The Dubna team were unable to detect any 0.3 s spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
activities.
The reaction was later studied in 2003 at the Paul Scherrer Institute
Paul Scherrer Institute
The Paul Scherrer Institute is a multi-disciplinary research institute which belongs to the Swiss ETH-Komplex covering also the ETH Zurich and EPFL...
(PSI) in Bern, Switzerland. They detected some spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
activities but were unable to confirm the formation of 259Rf.
248Cm(22Ne,αxn)266−xRf (x=3?)
This reaction was studied in 1999 at the University of Bern, Switzerland in order to search for the new isotope 263Rf. A rutherfordium fraction was separated and several SF events with long lifetimes and alpha decays with energy 7.8 MeV and 7.9 MeV were observed. A second experiment using a study of the fluoride of rutherfordium products also produced 7.9 MeV alpha decays.
248Cm(18O,xn)266−xRf (x=3?,5)
This reaction was first studied in 1970 by Albert Ghiorso at the LBNL. The team identified 261Rf in the 5n channel using the method of correlation of genetic parent-daughter decays. A half-life of 65 s was determined.
A repeat later that year using cation exchange chromatography indicated that the product did not form a +2 or +3 cation and behaved as eka-hafnium. A study of the properties of rutherfordium isotopes was performed in 1981 at the LBNL. In a series of reactions, a 1.5 s SF activity was identified and assigned to a fermium descendant although later evidence indicates a possible assignment to 262Rf. In contrast, in a subsequent review of isotope properties by Somerville et al. at LBNL in 1985, a 47 ms SF activity was assigned to 262Rf. This assignment has not been verified.
The reaction was further studied in 1991 by Czerwinski et al. at the LBNL. In this experiment, spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
activities with long lifetimes were observed in rutherfordium fractions and tentatively assigned to 263Rf. In 1996, chemical studies on the chloride of rutherfordium were published by the LBNL. In this experiment, the half-life was improved to 78 s. A repeat of the experiment in 2000 assessing the volatility of the bromide further refined the half-life to 75 s.
248Cm(16O,xn)264−xRf (x=4)
This reaction was studied in 1969 by Albert Ghiorso at the University of California. The aim was to detect the 0.1–0.3 s SF activity reported at Dubna, assigned to 260104. They were unable to do so, only observing a 10–30 ms SF activity, correctly assigned to 260104. The failure to observe the 0.3 s SF activity identified by Dubna gave the Americans the incentive to name this element rutherfordium.
246Cm(18O,xn)264−xRf
In an attempt to unravel the properties of spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
activities in the formation of rutherfordium isotopes, this reaction was performed in 1976 by the FLNR. They observed an 80 ms SF activity. Subsequent work led to the complete retraction of the 0.3s - 0.1s - 80 ms SF activities observed by the Dubna team and associated with background signals.
249Bk(15N,xn)264−xRf (x=4)
This reaction was studied in 1977 by the team in Dubna. They were able to confirm the detection of a 76 ms SF activity. The assignment to rutherfordium isotopes was later retracted. The LBNL re-studied the reaction in 1980 and in 1981 they reported that they were unable to confirm the ~80 ms SF activity. The Dubna team were able to measure a 28 ms SF activity in 1985 and assigned the isotope correctly to 260104.
249Cf(13C,xn)262−xRf (x=4)
This use of californium-249 as a target was first studied by Albert Ghiorso and the team at the University of California in 1969. They were able to observe an 11 ms SF activity which they correctly assigned to 258104.

In their 1969 discovery experiments, the team at University of California also used carbon-12
Carbon-12
Carbon-12 is the more abundant of the two stable isotopes of the element carbon, accounting for 98.89% of carbon; it contains 6 protons, 6 neutrons, and 6 electrons....
beam to irradiate a californium-249 target. They were able to confirm the 11 ms SF activity found with a carbon-13 beam and again correctly assigned to 258104. The actual discovery experiment was the observation of alpha decays genetically linked to 253102 and therefore positively identified as 257104.
In 1973, Bemis and his team at Oak Ridge confirmed the discovery by measuring coincident X-rays from the daughter 253102.
Synthesis of isotopes as decay products
Isotopes of rutherfordium have also been identified in the decay of heavier elements. Observations to date are summarized in the table below. EC refers to electron captureElectron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
.
| Evaporation residue | Observed Rf isotope |
|---|---|
| 288Uup | 268Rf (possible EC of 268Db) |
| 291Uuh, 287Uuq, 283Cn | 267Rf |
| 282Uut | 266Rf (EC of 266Db) |
| 269Sg | 265Rf |
| 271Hs | 263gRf |
| 263Db | 263mRf (EC of 263Db) |
| 266Sg (possibly 266mSg) | 262Rf (possibly 262mRf) |
| 277Cn, 273Ds, 269Hs, 265Sg | 261mRf, 261Rf |
| 271Ds, 267Hs, 263Sg | 259Rf |
| 269Ds, 265Hs, 261Sg | 257Rf |
| 264Hs, 260Sg | 256Rf |
| 259Sg | 255Rf |
Chronology of isotope discovery
| Isotope | Discovered | Reaction |
|---|---|---|
| 253Rf | 1994 | 204Pb(50Ti,n) |
| 254Rf | 1994 | 206Pb(50Ti,2n) |
| 255Rf | 1974? 1985 | 207Pb(50Ti,2n) |
| 256Rfg | 1974? 1985 | 208Pb(50Ti,2n) |
| 256Rfm1 | 2007 | 208Pb(50Ti,2n) |
| 256Rfm2 | 2007 | 208Pb(50Ti,2n) |
| 256Rfm3 | 2007 | 208Pb(50Ti,2n) |
| 257Rfg,m | 1969 | 249Cf(12C,4n) |
| 258Rf | 1969 | 249Cf(13C,4n) |
| 259Rf | 1969 | 249Cf(13C,3n) |
| 260Rf | 1969 | 248Cm(16O,4n) |
| 261Rfa | 1970 | 248Cm(18O,5n) |
| 261Rfb | 1996 | 208Pb(70Zn,n) |
| 262Rf | 1996 | 244Pu(22Ne,4n) |
| 263Rfa | 1990? | 248Cm(18O,3n) |
| 263Rfb | 2004 | 248Cm(26Mg,3n) |
| 264Rf | unknown | |
| 265Rf | unknown | |
| 266Rf? | 2006 | 237Np(48Ca,3n) |
| 267Rf | 2003/2004 | 238U(48Ca,3n) |
| 268Rf? | 2003 | 243Am(48Ca,3n) |
263Rf
Initial work on the synthesis of rutherfordium isotopes by hot fusion pathways focused on the synthesis of 263Rf. Several studies have indicated that this nuclide decays primarily by spontaneous fissionSpontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
with a long half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 10–20 minutes. Alpha particles with energy 7.8-7.9 MeV have also been associated with this nucleus. More recently, a study of hassium
Hassium
Hassium is a synthetic element with the symbol Hs and atomic number 108. It is the heaviest member of the group 8 elements. The element was first observed in 1984...
isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s allowed the synthesis of an atom of 263Rf decaying by spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
with a short half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 8 seconds. These two different decay modes must be associated with two isomeric states. Specific assignments are difficult due to the low number of observed events. It is reasonable to tentatively assign the long life to a meta-stable state, namely 263mRf, and the shorter life to the ground state, namely 263gRf. Further studies are required to allow a definite assignment.
261Rf
Early research on the synthesis of rutherfordium isotopes utilised the 244Pu(22Ne,5n)261Rf reaction. The product was found to undergo exclusive 8.28 MeV alpha decayAlpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 78 seconds. Later studies by the GSI
GSI
GSI may refer to:* Several geographical or geological organisations:** Geological Survey of India** Geological Survey of Iran** Geographical Society of Ireland** Geological Survey of Ireland** Geological Survey of Israel...
team on the synthesis of copernicium and hassium
Hassium
Hassium is a synthetic element with the symbol Hs and atomic number 108. It is the heaviest member of the group 8 elements. The element was first observed in 1984...
isotopes produced conflicting data. In this case, 261Rf was found to undergo 8.52 MeV alpha decay with a short half-life of 4 seconds. Later results indicated a predominant fission branch. These contradictions led to some doubt on the discovery of copernicium. However, it is now understood that the first nucleus belongs to an isomeric meta-stable state, namely 261mRf and the latter to the ground state isomer, namely 261gRf. The discovery and confirmation of 261gRf provided proof for the discovery of copernicium in 1996.
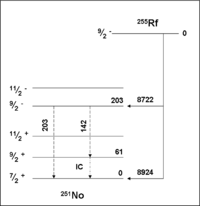
257Rf
A detailed spectroscopic study of the production of 257Rf nuclei using the reaction 208Pb(50Ti,n)257Rf allowed the identification of an isomeric level in 257Rf. The work confirmed that 257gRf has a very complicated spectrum with as many as 15 alpha lines. A level structure diagram was calculated for both isomers.257Rf

255Rf

268Rf
In the synthesis of ununpentiumUnunpentium
Ununpentium is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115....
, the isotope 288115 has been observed to decay to 268Db which undergoes spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
with a half-life of 29 hours. Given that the electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
of 268Db cannot be detected, these SF events may in fact be due to the SF of 268Rf, in which case the half-life of this isotope cannot be extracted.
266Rf
In the synthesis of ununtriumUnuntrium
Ununtrium is the temporary name of a synthetic element with the temporary symbol Uut and atomic number 113.It is placed as the heaviest member of the group 13 elements although a sufficiently stable isotope is not known at this time that would allow chemical experiments to confirm its position...
, the isotope 282113 has been observed to decay to 266Db which undergoes spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
with a half-life of 22 minutes. Given that the electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
of 266Db cannot be detected, these SF events may in fact be due to the SF of 266Rf, in which case the half-life of this isotope cannot be extracted.
265Rf
In 1999, American scientists at the University of California, Berkeley, announced that they has succeeded in synthesizing three atoms of 293118. These parent nuclei successively emitted seven alpha particles to form 265Rf nuclei. Their claim was retracted in 2001. As such, this rutherfordium isotope is unconfirmed or unknown.255mRf
A detailed spectroscopic study of the production of 255Rf nuclei using the reaction 206Pb(50Ti,n)255Rf allowed the tentative identification of an isomeric level in 255Rf. A more detailed study later confirmed that this was not the case.Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing rutherfordium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 50Ti | 208Pb | 258Rf | 38.0 nb, 17.0 MeV | 12.3 nb, 21.5 MeV | 660 pb, 29.0 MeV |
| 50Ti | 207Pb | 257Rf | 4.8 nb | ||
| 50Ti | 206Pb | 256Rf | 800 pb, 21.5 MeV | 2.4 nb, 21.5 MeV | |
| 50Ti | 204Pb | 254Rf | 190 pb, 15.6 MeV | ||
| 48Ti | 208Pb | 256Rf | 380 pb, 17.0 MeV |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing rutherfordium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.| Projectile | Target | CN | 3n | 4n | 5n |
|---|---|---|---|---|---|
| 26Mg | 238U | 264Rf | 240 pb | 1.1 nb | |
| 22Ne | 244Pu | 266Rf | + | 4.0 nb | |
| 18O | 248Cm | 266Rf | + | 13.0 nb |
Future experiments
The team at GSI are planning to perform first detailed spectroscopic studies on the isotope 259Rf. It will be produced in the new reaction: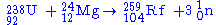
External links
- http://www.radiochemistry.org/periodictable/elements/isotopes_data/104.html
- http://www-phynu.cea.fr/science_en_ligne/carte_potentiels_microscopiques/choix/isotopes/z104_eng.html
- http://www.springermaterials.com/docs/info/978-3-540-70609-0_7730.html

