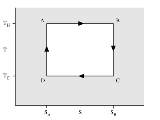
Isothermal process
Overview
An isothermal process is a change of a system, in which the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir (heat bath), and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir through heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
exchange. In contrast, an adiabatic process
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
is where a system exchanges no heat with its surroundings
Surroundings
Surroundings are the area around a given physical or geographical point or place. The exact definition depends on the field. Surroundings can also be used in geography and mathematics, as well as philosophy, with the literal or metaphorically extended definition.In thermodynamics, the term is used...
(Q = 0).
Discussions

