Isomerisation
Encyclopedia
In chemistry
isomerisation is the process by which one molecule
is transformed into another molecule which has exactly the same atoms, but the atoms are rearranged e.g. A-B-C → B-A-C (these related molecules are known as isomer
s ). In some molecules and under some conditions, isomerisation occurs spontaneously. Many isomers are equal or roughly equal in bond energy
, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerisation occurs intramolecular
ly it is considered a rearrangement reaction
.
An example of an organometallic
isomerisation is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.
The energy difference between two isomers is called isomerisation energy. Isomerisations with low energy difference both experimental and computational (in parentheses) are endothermic
trans-cis isomerisation of 2-butene
with 2.6 (1.2) kcal
/mol
, cracking of isopentane
to n-pentane with 3.6 (4.0) kcal/mol or conversion of trans-2-butene to 1-butene
with 2.6 (2.4) kcal/mol.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
isomerisation is the process by which one molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
is transformed into another molecule which has exactly the same atoms, but the atoms are rearranged e.g. A-B-C → B-A-C (these related molecules are known as isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s ). In some molecules and under some conditions, isomerisation occurs spontaneously. Many isomers are equal or roughly equal in bond energy
Bond energy
In chemistry, bond energy is the measure of bond strength in a chemical bond. It is the heat required to break one Mole of molecules into their individual atoms. For example, the carbon-hydrogen bond energy in methane E is the enthalpy change involved with breaking up one molecule of methane into...
, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerisation occurs intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
ly it is considered a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
.
An example of an organometallic
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
isomerisation is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.
Instances of Isomerisation
- Isomerisations in hydrocarbon crackingCracking (chemistry)In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products...
. This is usually employed in organic chemistryOrganic chemistryOrganic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, where fuels, such as pentanePentanePentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
, a straight-chain isomer, are heated in the presence of a platinum catalyst. The resulting mixture of straight- and branched-chain isomers then have to be separated. An industrial process is also the isomerisation of n-butane into isobutaneIsobutaneIsobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
.
-
- Trans-cis isomerism. In certain compounds an interconversion of cis and trans isomersGeometric isomerismIn organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
can be observed, for instance, with maleic acidMaleic acidMaleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
and with azobenzeneAzobenzeneAzobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
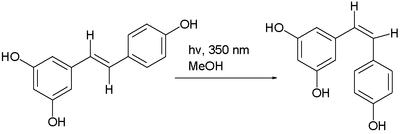
often by photoisomerization. An example is the photochemical conversion of the trans isomer to the cis isomer of resveratrolResveratrolResveratrol is a stilbenoid, a type of natural phenol, and a phytoalexin produced naturally by several plants when under attack by pathogens such as bacteria or fungi....
:
- Trans-cis isomerism. In certain compounds an interconversion of cis and trans isomers
- Aldose-ketose isomerism in biochemistry.
- Isomerisations between conformational isomers. These take place without an actual rearrangement for instance inconversion of two cyclohexane conformationCyclohexane conformationA cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds....
s - Fluxional molecules display rapid interconversion of isomers e.g. BullvaleneBullvaleneBullvalene is a hydrocarbon with the chemical formula C10H10 with the unusual property that the chemical bonds making up the molecule are constantly rearranging as in fluxional molecules...
. - valence isomerisation: the isomerisation of molecules which involve structural changes resulting only from a relocation of single and double bondDouble bondA double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s. If a dynamic equilibrium is established between the two isomers it is also referred to as valence tautomerism
The energy difference between two isomers is called isomerisation energy. Isomerisations with low energy difference both experimental and computational (in parentheses) are endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
trans-cis isomerisation of 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
with 2.6 (1.2) kcal
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, cracking of isopentane
Isopentane
Isopentane, C5H12, also called methylbutane or 2-methylbutane, is a branched-chain alkane with five carbon atoms. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure. The normal boiling point is just a few degrees above room temperature and...
to n-pentane with 3.6 (4.0) kcal/mol or conversion of trans-2-butene to 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
with 2.6 (2.4) kcal/mol.