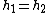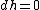Isenthalpic process
Encyclopedia
An isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy
, H; or specific enthalpy, h.
In a steady-state, steady-flow process, significant changes in pressure and temperature can occur to the fluid and yet the process will be isenthalpic if there is no transfer of heat to or from the surroundings, no work done on or by the surroundings, and no change in the kinetic energy of the fluid. (If a steady-state, steady-flow process is analysed using a control volume
everything outside the control volume is considered to be the surroundings.)
The throttling process
is a good example of an isenthalpic process. Consider the lifting of a relief valve or safety valve on a pressure vessel. The specific enthalpy of the fluid inside the pressure vessel is the same as the specific enthalpy of the fluid as it escapes from the valve. With a knowledge of the specific enthalpy of the fluid, and the pressure outside the pressure vessel, it is possible to determine the temperature and speed of the escaping fluid.
In an isenthalpic process:
Isenthalpic processes on an ideal gas
follow isotherms
since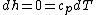 .
.
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
, H; or specific enthalpy, h.
In a steady-state, steady-flow process, significant changes in pressure and temperature can occur to the fluid and yet the process will be isenthalpic if there is no transfer of heat to or from the surroundings, no work done on or by the surroundings, and no change in the kinetic energy of the fluid. (If a steady-state, steady-flow process is analysed using a control volume
Control volume
In fluid mechanics and thermodynamics, a control volume is a mathematical abstraction employed in the process of creating mathematical models of physical processes. In an inertial frame of reference, it is a volume fixed in space or moving with constant velocity through which the fluid flows...
everything outside the control volume is considered to be the surroundings.)
The throttling process
Joule–Thomson effect
In thermodynamics, the Joule–Thomson effect or Joule–Kelvin effect or Kelvin–Joule effect describes the temperature change of a gas or liquid when it is forced through a valve or porous plug while kept insulated so that no heat is exchanged with the environment. This procedure is called a...
is a good example of an isenthalpic process. Consider the lifting of a relief valve or safety valve on a pressure vessel. The specific enthalpy of the fluid inside the pressure vessel is the same as the specific enthalpy of the fluid as it escapes from the valve. With a knowledge of the specific enthalpy of the fluid, and the pressure outside the pressure vessel, it is possible to determine the temperature and speed of the escaping fluid.
In an isenthalpic process:
Isenthalpic processes on an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
follow isotherms
Isothermal process
An isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
since
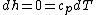 .
.