
Iron peak
Encyclopedia
The iron peak is a local maximum in the vicinity of Fe
(V
, Cr
, Mn
, Fe
, Co
and Ni
) on the graph of the abundances of chemical elements, as seen below.
For elements before iron
, nuclear fusion
releases energy. For elements heavier than iron, nuclear fusion
consumes energy, but nuclear fission
releases it. Chemical elements up to the iron peak are produced in ordinary stellar nucleosynthesis
. Heavier elements are produced only during supernova nucleosynthesis
. This is why we have more iron peak elements than in its neighbourhood.
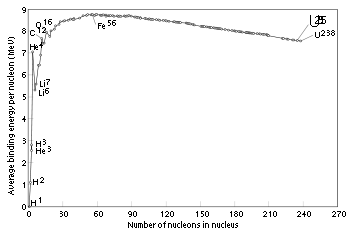
As can be seen, light elements such as hydrogen release large amounts of energy (a big increase in binding energy) as nucleons are added—the process of fusion. Conversely, heavy elements such as uranium release energy when nucleons are removed—the process of nuclear fission
. Although nuclei with 58 and 62 nucleons have the very highest binding energy, fusing four nucleons to nickel–56 to produce the next element — zinc–60 — actually requires energy rather than releasing any. Accordingly, nickel–56 is the last fusion product produced in the cores of high-mass stars (see also Silicon burning process
).
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
(V
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
, Cr
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
, Mn
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
, Fe
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, Co
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
and Ni
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
) on the graph of the abundances of chemical elements, as seen below.
For elements before iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
releases energy. For elements heavier than iron, nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
consumes energy, but nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
releases it. Chemical elements up to the iron peak are produced in ordinary stellar nucleosynthesis
Stellar nucleosynthesis
Stellar nucleosynthesis is the collective term for the nuclear reactions taking place in stars to build the nuclei of the elements heavier than hydrogen. Some small quantity of these reactions also occur on the stellar surface under various circumstances...
. Heavier elements are produced only during supernova nucleosynthesis
Supernova nucleosynthesis
Supernova nucleosynthesis is the production of new chemical elements inside supernovae. It occurs primarily due to explosive nucleosynthesis during explosive oxygen burning and silicon burning...
. This is why we have more iron peak elements than in its neighbourhood.
Binding energy
The graph below shows the binding energy of various elements. Increasing values of binding energy can be thought of in two ways:- It is the energy required to remove a nucleonNucleonIn physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
from a nucleus; and - It is the energy released when a nucleon is added to a nucleus.
As can be seen, light elements such as hydrogen release large amounts of energy (a big increase in binding energy) as nucleons are added—the process of fusion. Conversely, heavy elements such as uranium release energy when nucleons are removed—the process of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. Although nuclei with 58 and 62 nucleons have the very highest binding energy, fusing four nucleons to nickel–56 to produce the next element — zinc–60 — actually requires energy rather than releasing any. Accordingly, nickel–56 is the last fusion product produced in the cores of high-mass stars (see also Silicon burning process
Silicon burning process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main...
).


