
Intensive and extensive properties
Encyclopedia
In the physical science
s, an intensive property (also called a bulk property, intensive quantity, or intensive variable), is a physical property
of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.
By contrast, an extensive property (also extensive quantity, extensive variable, or extensive parameter) is one that is additive for independent, noninteracting subsystems. It is directly proportional to the amount of material in the system.
For example, density
is an intensive property of a substance because it does not depend on the amount of that substance; mass
and volume, which are measures of the amount of the substance, are extensive properties.
Note that the ratio of two extensive properties that scale in the same way is scale-invariant, and hence an intensive property.
whose value does not depend on the amount of the substance for which it is measured. For example, the temperature
of a system in thermal equilibrium is the same as the temperature of any part of it. If the system is divided the temperature of each subsystem is identical. The same applies to the density
of a homogeneous system: if the system is divided in half, the mass and the volume change in the identical ratio and the density remains unchanged.
According to the state postulate
, for a sufficiently simple system, only two independent intensive variables are needed to fully specify the entire state of a system. Other intensive properties can be derived from the two known values.
Some intensive properties, such as viscosity
, are empirical
macroscopic
quantities
and are not relevant to extremely small systems.
If a set of parameters,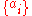 , are intensive properties and another set,
, are intensive properties and another set, 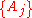 , are extensive properties, then the function
, are extensive properties, then the function 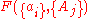 is an intensive property if for all
is an intensive property if for all  ,
,
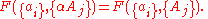
It follows, for example, that the ratio
of two extensive properties is an intensive property - density (intensive) is equal to mass (extensive) divided by volume (extensive).
and the properties of the resulting solution
are not a simple combination of the properties of its constituents.] Let V be an intensive variable. The value of variable V corresponding to the first substance is Va, and the value of V corresponding to the second substance is Vb. If the two pieces a and b are put together, forming a piece of substance "a+b" of amount ma+b = ma+mb, then the value of their intensive variable V is:

which is a weighted mean
. Further, if Va = Vb then Va + b = Va = Vb, i.e. the intensive variable is independent of the amount. Note that this property holds only as long as other variables on which the intensive variable depends stay constant.
In a thermodynamic system
composed of two monatomic ideal gas
es, a and b, if the two gases are mixed, the final temperature T is
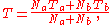
a weighted mean where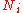 is the number of particles in gas i, and
is the number of particles in gas i, and 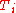 is the corresponding temperature.
is the corresponding temperature.
Note that you have to measure the amounts in the same unit that was used to calculate the intensive property from the extensive property. So when you interpolate density, you have to measure the properties in volume, as density is mass per volume. The formula makes no sense when you measure the properties in mass (kg).
as a physical quantity which is the sum of the properties of separate noninteracting subsystems that compose the entire system. The value of such a property is proportional to the size of the system
it describes, or to the quantity of matter in the system.
Extensive properties are the counterparts of intensive properties, which are intrinsic to a particular subsystem. Dividing one type of extensive property by a different type of extensive property will in general give an intensive value. For example, mass
(extensive) divided by volume (extensive) gives density
(intensive).
 are intensive properties and another set
are intensive properties and another set 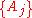 are extensive properties, then the function
are extensive properties, then the function  is an extensive property if for all
is an extensive property if for all  ,
,
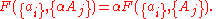
Thus, extensive properties are homogeneous function
s (of degree 1) with respect to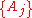 . It follows from Euler's homogeneous function theorem that
. It follows from Euler's homogeneous function theorem that

where the partial derivative
is taken with all parameters constant except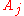 . The converse is also true - any function which obeys the above relationship will be extensive.
. The converse is also true - any function which obeys the above relationship will be extensive.
in question, include volume (V), internal energy (U), enthalpy (H), entropy (S), Gibbs free energy (G), Helmholtz free energy (A), and heat capacities (Cv and Cp) (in the sense of thermal mass
). Note that the main symbols of these extensive thermodynamic properties shown here are capital letters. Except for volume (V), these extensive properties are dependent on the amount of material (substance) in the thermodynamic system in question.
For homogeneous substances, these extensive thermodynamic properties each have analogous intensive thermodynamic properties, which can be expressed on a per mass basis, and the corresponding intensive property symbols would be the lower case letters of the corresponding extensive property. Examples of intensive thermodynamic properties, which are independent on the size of the thermodynamic system in question and are analogous to the extensive ones mentioned above, include specific volume (v), specific internal energy (u), specific enthalpy (h), specific entropy (s), specific Gibbs free energy (g), specific Helmholtz free energy (a), and specific heat capacities (cv and cp, sometimes simply called specific heats). These intensive thermodynamic properties are effectively material properties which are valid at a point in a thermodynamic system or at a point in space at a certain time. These intensive properties are dependent on the conditions at that point such as temperature, pressure, and material composition, but are not considered dependent on the size of a thermodynamic system or on the amount of material in the system. See the table below. Specific volume is volume per mass, the reciprocal
of density
which equals mass per volume.
If a molecular weight can be assigned for the substance, or the number of moles in the system can be determined, then each of these thermodynamic properties can be expressed on a per mole basis. These intensive properties could be named after the analogous extensive properties but with the word "molar" preceding them; thus molar volume, molar internal energy, molar enthalpy, molar entropy, etc. Although the same small letters can be used as in the analogous specific properties indicating they are intensive, sometimes the corresponding capital letters have been used (and understood to be on a per mole basis), and there seems to be no universally agreed upon symbol convention for these molar properties. A well known molar volume is that of an ideal gas
at STP (Standard Temperature and Pressure); this molar volume = 22.41 liter
s per mole. Molar Gibbs free energy is commonly referred to as chemical potential
, symbolized by μ, particularly when discussing a partial molar Gibbs free energy μi for a component i in a mixture.
of two resistors is the sum of their two resistances only if the resistors are connected in series
, but not if they are connected in parallel. The electrical resistance of independent noninteracting resistors (subsystems) is therefore not additive in general, and electrical resistance is not an extensive property. Nor is it intensive because the resistance of the two resistors is not equal in general, even if they consist of the same material at the same temperature and pressure.
Physical science
Physical science is an encompassing term for the branches of natural science and science that study non-living systems, in contrast to the life sciences...
s, an intensive property (also called a bulk property, intensive quantity, or intensive variable), is a physical property
Physical property
A physical property is any property that is measurable whose value describes a physical system's state. The changes in the physical properties of a system can be used to describe its transformations ....
of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.
By contrast, an extensive property (also extensive quantity, extensive variable, or extensive parameter) is one that is additive for independent, noninteracting subsystems. It is directly proportional to the amount of material in the system.
For example, density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
is an intensive property of a substance because it does not depend on the amount of that substance; mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
and volume, which are measures of the amount of the substance, are extensive properties.
Note that the ratio of two extensive properties that scale in the same way is scale-invariant, and hence an intensive property.
Intensive properties
An intensive property is a physical quantityPhysical quantity
A physical quantity is a physical property of a phenomenon, body, or substance, that can be quantified by measurement.-Definition of a physical quantity:Formally, the International Vocabulary of Metrology, 3rd edition defines quantity as:...
whose value does not depend on the amount of the substance for which it is measured. For example, the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of a system in thermal equilibrium is the same as the temperature of any part of it. If the system is divided the temperature of each subsystem is identical. The same applies to the density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of a homogeneous system: if the system is divided in half, the mass and the volume change in the identical ratio and the density remains unchanged.
According to the state postulate
State postulate
The state postulate is a term used in thermodynamics that defines the given number of properties to a thermodynamic system in a state of equilibrium. The state postulate allows a certain number of properties to be specified to place it in thermodynamic equilibrium...
, for a sufficiently simple system, only two independent intensive variables are needed to fully specify the entire state of a system. Other intensive properties can be derived from the two known values.
Some intensive properties, such as viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
, are empirical
Empirical
The word empirical denotes information gained by means of observation or experimentation. Empirical data are data produced by an experiment or observation....
macroscopic
Macroscopic
The macroscopic scale is the length scale on which objects or processes are of a size which is measurable and observable by the naked eye.When applied to phenomena and abstract objects, the macroscopic scale describes existence in the world as we perceive it, often in contrast to experiences or...
quantities
Quantity
Quantity is a property that can exist as a magnitude or multitude. Quantities can be compared in terms of "more" or "less" or "equal", or by assigning a numerical value in terms of a unit of measurement. Quantity is among the basic classes of things along with quality, substance, change, and relation...
and are not relevant to extremely small systems.
Combined intensive properties
There are four properties in any thermodynamic system, two intensive ones and two extensive ones.If a set of parameters,
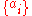 , are intensive properties and another set,
, are intensive properties and another set, 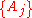 , are extensive properties, then the function
, are extensive properties, then the function 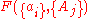 is an intensive property if for all
is an intensive property if for all  ,
,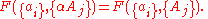
It follows, for example, that the ratio
Ratio
In mathematics, a ratio is a relationship between two numbers of the same kind , usually expressed as "a to b" or a:b, sometimes expressed arithmetically as a dimensionless quotient of the two which explicitly indicates how many times the first number contains the second In mathematics, a ratio is...
of two extensive properties is an intensive property - density (intensive) is equal to mass (extensive) divided by volume (extensive).
Joining systems
Let there be a system or piece of substance a of amount ma and another piece of substance b of amount mb which can be combined without interaction. [For example, lead and tin combine without interaction, but common salt dissolves in waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and the properties of the resulting solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
are not a simple combination of the properties of its constituents.] Let V be an intensive variable. The value of variable V corresponding to the first substance is Va, and the value of V corresponding to the second substance is Vb. If the two pieces a and b are put together, forming a piece of substance "a+b" of amount ma+b = ma+mb, then the value of their intensive variable V is:

which is a weighted mean
Weighted mean
The weighted mean is similar to an arithmetic mean , where instead of each of the data points contributing equally to the final average, some data points contribute more than others...
. Further, if Va = Vb then Va + b = Va = Vb, i.e. the intensive variable is independent of the amount. Note that this property holds only as long as other variables on which the intensive variable depends stay constant.
In a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
composed of two monatomic ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
es, a and b, if the two gases are mixed, the final temperature T is
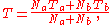
a weighted mean where
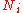 is the number of particles in gas i, and
is the number of particles in gas i, and 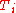 is the corresponding temperature.
is the corresponding temperature.Note that you have to measure the amounts in the same unit that was used to calculate the intensive property from the extensive property. So when you interpolate density, you have to measure the properties in volume, as density is mass per volume. The formula makes no sense when you measure the properties in mass (kg).
Examples
Examples of intensive properties include:- temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
- chemical potentialChemical potentialChemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
- densityDensityThe mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
(or specific gravitySpecific gravitySpecific gravity is the ratio of the density of a substance to the density of a reference substance. Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance. The reference substance is nearly always water for...
) - viscosityViscosityViscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
- velocityVelocityIn physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
- electrical resistivity
- spectral absorption maxima (in solutionSolutionIn chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
) - specific energySpecific energySpecific energy is defined as the energy per unit mass. Common metric units are J/kg. It is an intensive property. Contrast this with energy, which is an extensive property. There are two main types of specific energy: potential energy and specific kinetic energy. Others are the gray and sievert,...
- specific heat capacityHeat capacityHeat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
- hardness
- melting pointMelting pointThe melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
and boiling pointBoiling pointThe boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid.... - pressurePressurePressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
- ductilityDuctilityIn materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
- elasticityElasticity (physics)In physics, elasticity is the physical property of a material that returns to its original shape after the stress that made it deform or distort is removed. The relative amount of deformation is called the strain....
- malleability
- magnetizationMagnetizationIn classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
- concentrationConcentrationIn chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
Extensive properties
An extensive property is defined by the IUPAC Green BookIUPAC green book
right|thumb|Front cover of the Green BookQuantities, Units and Symbols in Physical Chemistry Third Edition , also known as the Green Book, published in Aug 2007 by IUPAC is based on the most up to date sources for fundamental constants, data and nomenclature in the field of chemistry and physics...
as a physical quantity which is the sum of the properties of separate noninteracting subsystems that compose the entire system. The value of such a property is proportional to the size of the system
System
System is a set of interacting or interdependent components forming an integrated whole....
it describes, or to the quantity of matter in the system.
Extensive properties are the counterparts of intensive properties, which are intrinsic to a particular subsystem. Dividing one type of extensive property by a different type of extensive property will in general give an intensive value. For example, mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
(extensive) divided by volume (extensive) gives density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
(intensive).
Combined extensive properties
If a set of parameters are intensive properties and another set
are intensive properties and another set 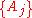 are extensive properties, then the function
are extensive properties, then the function  is an extensive property if for all
is an extensive property if for all  ,
,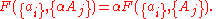
Thus, extensive properties are homogeneous function
Homogeneous function
In mathematics, a homogeneous function is a function with multiplicative scaling behaviour: if the argument is multiplied by a factor, then the result is multiplied by some power of this factor. More precisely, if is a function between two vector spaces over a field F, and k is an integer, then...
s (of degree 1) with respect to
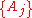 . It follows from Euler's homogeneous function theorem that
. It follows from Euler's homogeneous function theorem that
where the partial derivative
Partial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant...
is taken with all parameters constant except
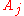 . The converse is also true - any function which obeys the above relationship will be extensive.
. The converse is also true - any function which obeys the above relationship will be extensive.Examples
Examples of extensive properties include:- energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
- entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
- Gibbs energy
- massMassMass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
- particle numberParticle numberThe particle number of a thermodynamic system, conventionally indicated with the letter N, is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle...
- momentumMomentumIn classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
- number of moles
- volume
Related extensive and intensive properties
Although not true for all physical properties, there are a number of properties which have corresponding extensive and intensive analogs, many of which are thermodynamic properties. Examples of such extensive thermodynamic properties, which are dependent on the size of the thermodynamic systemThermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
in question, include volume (V), internal energy (U), enthalpy (H), entropy (S), Gibbs free energy (G), Helmholtz free energy (A), and heat capacities (Cv and Cp) (in the sense of thermal mass
Thermal mass
Thermal mass is a concept in building design which describes how the mass of the building provides "inertia" against temperature fluctuations, sometimes known as the thermal flywheel effect...
). Note that the main symbols of these extensive thermodynamic properties shown here are capital letters. Except for volume (V), these extensive properties are dependent on the amount of material (substance) in the thermodynamic system in question.
For homogeneous substances, these extensive thermodynamic properties each have analogous intensive thermodynamic properties, which can be expressed on a per mass basis, and the corresponding intensive property symbols would be the lower case letters of the corresponding extensive property. Examples of intensive thermodynamic properties, which are independent on the size of the thermodynamic system in question and are analogous to the extensive ones mentioned above, include specific volume (v), specific internal energy (u), specific enthalpy (h), specific entropy (s), specific Gibbs free energy (g), specific Helmholtz free energy (a), and specific heat capacities (cv and cp, sometimes simply called specific heats). These intensive thermodynamic properties are effectively material properties which are valid at a point in a thermodynamic system or at a point in space at a certain time. These intensive properties are dependent on the conditions at that point such as temperature, pressure, and material composition, but are not considered dependent on the size of a thermodynamic system or on the amount of material in the system. See the table below. Specific volume is volume per mass, the reciprocal
Multiplicative inverse
In mathematics, a multiplicative inverse or reciprocal for a number x, denoted by 1/x or x−1, is a number which when multiplied by x yields the multiplicative identity, 1. The multiplicative inverse of a fraction a/b is b/a. For the multiplicative inverse of a real number, divide 1 by the...
of density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
which equals mass per volume.
| Extensive property |
Symbol | SI units | Intensive property** |
Symbol | SI units |
|---|---|---|---|---|---|
| Volume | |
Litér - External links :*... * |
Specific volume Specific volume In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass. It is the reciprocal of density:In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass... *** |
|
Kilogram The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water... or l*/kg |
| Internal energy Internal energy In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal... |
|
Joule The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second... |
Specific internal energy | |
|
| Entropy Entropy Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when... |
|
Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... |
Specific entropy | |
|
| Enthalpy Enthalpy Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a... |
|
Specific enthalpy | |
||
| Gibbs free energy Gibbs free energy In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure... |
|
Specific Gibbs free energy | |
||
| Heat capacity Heat capacity Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount... at constant volume |
|
Specific heat capacity at constant volume |
|
||
| Heat capacity Heat capacity Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount... at constant pressure |
|
Specific heat capacity at constant pressure |
|
- * l = literLitér- External links :*...
, J = jouleJouleThe joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second... - ** specific properties, expressed on a per mass basis
- *** Specific volume is the reciprocalMultiplicative inverseIn mathematics, a multiplicative inverse or reciprocal for a number x, denoted by 1/x or x−1, is a number which when multiplied by x yields the multiplicative identity, 1. The multiplicative inverse of a fraction a/b is b/a. For the multiplicative inverse of a real number, divide 1 by the...
of densityDensityThe mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
.
If a molecular weight can be assigned for the substance, or the number of moles in the system can be determined, then each of these thermodynamic properties can be expressed on a per mole basis. These intensive properties could be named after the analogous extensive properties but with the word "molar" preceding them; thus molar volume, molar internal energy, molar enthalpy, molar entropy, etc. Although the same small letters can be used as in the analogous specific properties indicating they are intensive, sometimes the corresponding capital letters have been used (and understood to be on a per mole basis), and there seems to be no universally agreed upon symbol convention for these molar properties. A well known molar volume is that of an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
at STP (Standard Temperature and Pressure); this molar volume = 22.41 liter
Litér
- External links :*...
s per mole. Molar Gibbs free energy is commonly referred to as chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
, symbolized by μ, particularly when discussing a partial molar Gibbs free energy μi for a component i in a mixture.
Counter-example
There are measured physical properties which are neither intensive nor extensive. For example the electrical resistanceElectrical resistance
The electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes. Electrical resistance shares some conceptual parallels with the mechanical...
of two resistors is the sum of their two resistances only if the resistors are connected in series
Series and parallel circuits
Components of an electrical circuit or electronic circuit can be connected in many different ways. The two simplest of these are called series and parallel and occur very frequently. Components connected in series are connected along a single path, so the same current flows through all of the...
, but not if they are connected in parallel. The electrical resistance of independent noninteracting resistors (subsystems) is therefore not additive in general, and electrical resistance is not an extensive property. Nor is it intensive because the resistance of the two resistors is not equal in general, even if they consist of the same material at the same temperature and pressure.

