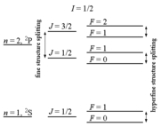
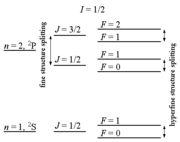
Hyperfine structure
Encyclopedia
The term hyperfine structure refers to a collection of different effects leading to small shifts and splittings in the energy level
s of atoms, molecule
s and ion
s. The name is a reference to the fine structure
which results from the interaction between the magnetic moment
s associated with electron spin and the electrons' orbital angular momentum
. Hyperfine structure, with energy shifts typically orders of magnitude smaller than the fine structure, results from the interactions of the nucleus
(or nuclei, in molecules) with internally generated electric and magnetic fields.
In atoms, hyperfine structure occurs due to the energy of the nuclear magnetic dipole moment
in the magnetic field
generated by the electrons, and the energy of the nuclear electric quadrupole moment
in the electric field gradient
due to the distribution of charge within the atom. Molecular hyperfine structure is generally dominated by these two effects, but also includes the energy associated with the interaction between the magnetic moments associated with different magnetic nuclei in a molecule, as well as between the nuclear magnetic moments and the magnetic field generated by the rotation of the molecule.
. It could, however, only be explained in terms of quantum mechanics when Wolfgang Pauli
proposed the existence of a small nuclear magnetic moment in 1924.
In 1935, M. Schüler and Theodor Schmidt proposed the existence of a nuclear quadrupole moment in order to explain anomalies in the hyperfine structure.
, consisting of the interaction of the nuclear multipole moments
(excluding the electric monopole) with internally generated fields. The theory is derived first for the atomic case, but can be applied to each nucleus in a molecule. Following this there is a discussion of the additional effects unique to the molecular case.
is typically the magnetic dipole term. Atomic nuclei with a non-zero nuclear spin have a magnetic dipole moment, given by:
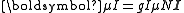 .
.
There is an energy associated with a magnetic dipole moment in the presence of a magnetic field. For a nuclear magnetic dipole moment, μI, placed in a magnetic field, B, the relevant term in the Hamiltonian is given by:
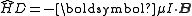 .
.
In the absence of an externally applied field, the magnetic field experienced by the nucleus is that associated with the orbital (l) and spin (s) angular momentum of the electrons:
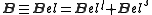 .
.
Electron orbital angular momentum results from the motion of the electron about some fixed external point that we shall take to be the location of the nucleus. The magnetic field at the nucleus due to the motion of a single electron, with charge -e
at a position r relative to the nucleus, is given by:
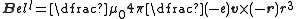 ,
,
where -r gives the position of the nucleus relative to the electron. Written in terms of the Bohr magneton, this gives:
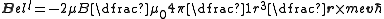 .
.
Recognizing that mev is the electron momentum, p, and that r×p/ħ is the orbital angular momentum
in units of ħ, l, we can write:
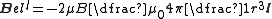 .
.
For a many electron atom this expression is generally written in terms of the total orbital angular momentum,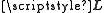 , by summing over the electrons and using the projection operator,
, by summing over the electrons and using the projection operator, 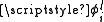 , where
, where 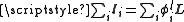 . For states with a well defined projection of the orbital angular momentum, Lz, we can write
. For states with a well defined projection of the orbital angular momentum, Lz, we can write 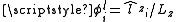 , giving:
, giving:
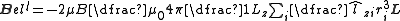 .
.
The electron spin angular momentum is a fundamentally different property that is intrinsic to the particle and therefore does not depend on the motion of the electron. Nonetheless it is angular momentum and any angular momentum associated with a charged particle results in a magnetic dipole moment, which is the source of a magnetic field. An electron with spin angular momentum, s, has a magnetic moment, μs, given by:
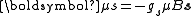 ,
,
where gs is the electron spin g-factor and the negative sign is because the electron is negatively charged (consider that negatively and positively charged particles with identical mass, travelling on equivalent paths, would have the same angular momentum, but would result in currents in the opposite direction).
The magnetic field of a dipole moment, μs, is given by:
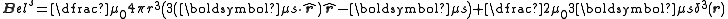 .
.
The complete magnetic dipole contribution to the hyperfine Hamiltonian is thus given by:
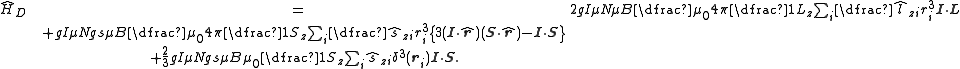
The first term gives the energy of the nuclear dipole in the field due to the electronic orbital angular momentum. The second term gives the energy of the "finite distance" interaction of the nuclear dipole with the field due to the electron spin magnetic moments. The final term, often known as the "Fermi contact
" term relates to the direct interaction of the nuclear dipole with the spin dipoles and is only non-zero for states with a finite electron spin density at the position of the nucleus (those with unpaired electrons in s-subshells). It has been argued that one may get a different expression when taking into account the detailed nuclear dipole moment distribution.
For states with l ≠ 0 this can be expressed in the form
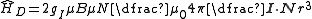 ,
,
where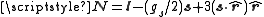 .
.
If hyperfine structure is small compared with the fine structure (sometimes called IJ-coupling by analogy with LS-coupling), I and J are good quantum number
s and matrix elements of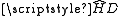 can be approximated as diagonal in I and J. In this case (generally true for light elements), we can project N onto J (where J = L + S is the total electronic angular momentum) and we have:
can be approximated as diagonal in I and J. In this case (generally true for light elements), we can project N onto J (where J = L + S is the total electronic angular momentum) and we have:
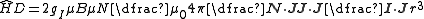 .
.
This is commonly written as
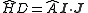 ,
,
with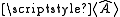 determined by experiment. Since I.J = ½{F.F - I.I - J.J} (where F = I + J is the total angular momentum), this gives an energy of
determined by experiment. Since I.J = ½{F.F - I.I - J.J} (where F = I + J is the total angular momentum), this gives an energy of
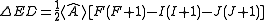 .
.
In this case the hyperfine interaction satisfies the Lande interval rule
.
 have an electric quadrupole moment. In the general case this is represented by a rank-2 tensor
have an electric quadrupole moment. In the general case this is represented by a rank-2 tensor
,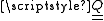 , with components given by:
, with components given by:
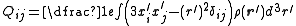 ,
,
where i and j are the tensor indices running from 1 to 3, xi and xj are the spatial variables x, y and z depending on the values of i and j respectively, δij is the Kronecker delta and ρ(r) is the charge density. Being a 3-dimensional rank-2 tensor, the quadrupole moment has 32 = 9 components. From the definition of the components it is clear that the quadrupole tensor is a symmetric matrix (Qij = Qji) that is also traceless (ΣiQii = 0), giving only five components in the irreducible representation. Expressed using the notation of irreducible spherical tensors we have:
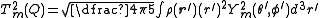 .
.
The energy associated with an electric quadrupole moment in an electric field depends not on the field strength, but on the electric field gradient, confusingly labelled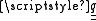 , another rank-2 tensor given by the outer product
, another rank-2 tensor given by the outer product
of the del operator with the electric field vector:
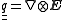 ,
,
with components given by:
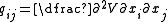 .
.
Again it is clear this is a symmetric matrix and, because the source of the electric field at the nucleus is a charge distribution entirely outside the nucleus, this can be expressed as a 5-component spherical tensor,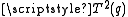 , with:
, with:
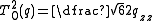
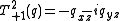
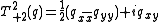 ,
,
where:
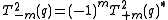 .
.
The quadrupolar term in the Hamiltonian is thus given by:
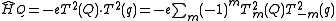 .
.
A typical atomic nucleus closely approximates cylindrical symmetry and therefore all off-diagonal elements are close to zero. For this reason the nuclear electric quadrupole moment is often represented by Qzz.
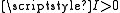 and an electric quadrupole term for each nucleus with
and an electric quadrupole term for each nucleus with 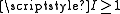 . The magnetic dipole terms were first derived for diatomic molecules by Frosch and Foley and the resulting hyperfine parameters are often called the Frosch and Foley parameters.
. The magnetic dipole terms were first derived for diatomic molecules by Frosch and Foley and the resulting hyperfine parameters are often called the Frosch and Foley parameters.
In addition to the effects described above there are a number of effects specific to the molecular case.
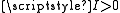 has a non-zero magnetic moment that is both the source of a magnetic field and has an associated energy due to the presence of the combined field of all of the other nuclear magnetic moments. A summation over each magnetic moment dotted with the field due to each other magnetic moment gives the direct nuclear spin-spin term in the hyperfine Hamiltonian,
has a non-zero magnetic moment that is both the source of a magnetic field and has an associated energy due to the presence of the combined field of all of the other nuclear magnetic moments. A summation over each magnetic moment dotted with the field due to each other magnetic moment gives the direct nuclear spin-spin term in the hyperfine Hamiltonian, 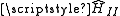 .
.
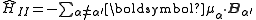 ,
,
where α and α‘ are indices representing the nucleus contributing to the energy and the nucleus that is the source of the field respectively. Substituting in the expressions for the dipole moment in terms of the nuclear angular momentum and the magnetic field of a dipole, both given above, we have:
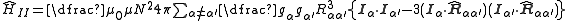 .
.
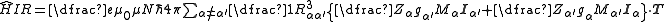
spectra of free radicals and transition-metal ions
.
Hyperfine structure gives the 21 cm line observed in H I region
s in interstellar medium
.
Carl Sagan
and Frank Drake
considered the hyperfine transition of hydrogen to be a sufficiently universal phenomenon so as to be used as a base unit of time and length on the Pioneer plaque
and later Voyager Golden Record
.
In radio astronomy
, heterodyne receiver
s are widely used in detection of the electromagnetic signals from celestial objects. The separations among various components of a hyperfine structure are usually small enough to fit into the receiver's IF
band. Because optical depth
varies with frequency, strength ratios among the hyperfine components differ from that of their intrinsic intensities. From this we can derive the object's physical parameters.
process uses the hyperfine splitting between optical transitions in uranium-235 and uranium-238 to selectively photo-ionize only the uranium-235 atoms and then separate the ionized particles from the non-ionized ones. Precisely tuned dye laser
s are used as the sources of the necessary exact wavelength radiation.
notch filter with very high stability, repeatability and Q factor
, which can thus be used as a basis for very precise atomic clock
s. Typically, the hyperfine structure transition frequency of a particular isotope of caesium
or rubidium
atoms is used as a basis for these clocks.
Due to the accuracy of hyperfine structure transition-based atomic clocks, they are now used as the basis for the definition of the second. One second
is now defined to be exactly 9,192,631,770 cycles of the hyperfine structure transition frequency of caesium-133 atoms.
Since 1983, the meter is defined by declaring the speed of light in a vacuum to be exactly 299,792,458 metres per second. Thus:
The metre is the length of the path travelled by light in vacuum during a time interval of 1/299,792,458 of a second.
have been used to measure the value of the fine structure constant α. Comparison with measurements of α in other physical systems provides a stringent test of QED
.
are commonly used for storing qubit
s in ion-trap quantum computing. They have the advantage of having very long lifetimes, experimentally exceeding ~10 min (compared to ~1 s for metastable electronic levels).
The frequency associated with the states' energy separation is in the microwave
region, making it possible to drive hyperfine transitions using microwave radiation. However, at present no emitter is available that can be focused to address a particular ion from a sequence. Instead, a pair of laser
pulses can be used to drive the transition, by having their frequency difference (detuning) equal to the required transition's frequency. This is essentially a stimulated Raman transition.
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
s of atoms, molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s and ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. The name is a reference to the fine structure
Fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to first order relativistic corrections.The gross structure of line spectra is the line spectra predicted by non-relativistic electrons with no spin. For a hydrogenic atom, the gross structure energy...
which results from the interaction between the magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
s associated with electron spin and the electrons' orbital angular momentum
Azimuthal quantum number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital...
. Hyperfine structure, with energy shifts typically orders of magnitude smaller than the fine structure, results from the interactions of the nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
(or nuclei, in molecules) with internally generated electric and magnetic fields.
In atoms, hyperfine structure occurs due to the energy of the nuclear magnetic dipole moment
Nuclear magnetic moment
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is mainly a magnetic dipole moment; the quadrupole moment does cause some small shifts in the hyperfine structure as well....
in the magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
generated by the electrons, and the energy of the nuclear electric quadrupole moment
Quadrupole
A quadrupole or quadrapole is one of a sequence of configurations of—for example—electric charge or current, or gravitational mass that can exist in ideal form, but it is usually just part of a multipole expansion of a more complex structure reflecting various orders of complexity.-Mathematical...
in the electric field gradient
Electric field gradient
In atomic, molecular, and solid-state physics, the electric field gradient measures the rate of change of the electric field at an atomic nucleus generated by the electronic charge distribution and the other nuclei...
due to the distribution of charge within the atom. Molecular hyperfine structure is generally dominated by these two effects, but also includes the energy associated with the interaction between the magnetic moments associated with different magnetic nuclei in a molecule, as well as between the nuclear magnetic moments and the magnetic field generated by the rotation of the molecule.

History
The optical hyperfine structure was already observed in 1881 by Albert Abraham MichelsonAlbert Abraham Michelson
Albert Abraham Michelson was an American physicist known for his work on the measurement of the speed of light and especially for the Michelson-Morley experiment. In 1907 he received the Nobel Prize in Physics...
. It could, however, only be explained in terms of quantum mechanics when Wolfgang Pauli
Wolfgang Pauli
Wolfgang Ernst Pauli was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after being nominated by Albert Einstein, he received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or...
proposed the existence of a small nuclear magnetic moment in 1924.
In 1935, M. Schüler and Theodor Schmidt proposed the existence of a nuclear quadrupole moment in order to explain anomalies in the hyperfine structure.
Theory
The theory of hyperfine structure comes directly from electromagnetismElectromagnetism
Electromagnetism is one of the four fundamental interactions in nature. The other three are the strong interaction, the weak interaction and gravitation...
, consisting of the interaction of the nuclear multipole moments
Multipole moments
In mathematics, especially as applied to physics, multipole moments are the coefficients of a series expansion of a potential due to continuous or discrete sources . A multipole moment usually involves powers of the distance to the origin, as well as some angular dependence...
(excluding the electric monopole) with internally generated fields. The theory is derived first for the atomic case, but can be applied to each nucleus in a molecule. Following this there is a discussion of the additional effects unique to the molecular case.
Magnetic dipole
The dominant term in the hyperfine HamiltonianHamiltonian (quantum mechanics)
In quantum mechanics, the Hamiltonian H, also Ȟ or Ĥ, is the operator corresponding to the total energy of the system. Its spectrum is the set of possible outcomes when one measures the total energy of a system...
is typically the magnetic dipole term. Atomic nuclei with a non-zero nuclear spin have a magnetic dipole moment, given by:
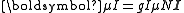 .
.There is an energy associated with a magnetic dipole moment in the presence of a magnetic field. For a nuclear magnetic dipole moment, μI, placed in a magnetic field, B, the relevant term in the Hamiltonian is given by:
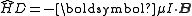 .
.In the absence of an externally applied field, the magnetic field experienced by the nucleus is that associated with the orbital (l) and spin (s) angular momentum of the electrons:
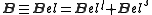 .
.Electron orbital angular momentum results from the motion of the electron about some fixed external point that we shall take to be the location of the nucleus. The magnetic field at the nucleus due to the motion of a single electron, with charge -e
Elementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
at a position r relative to the nucleus, is given by:
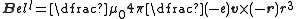 ,
,where -r gives the position of the nucleus relative to the electron. Written in terms of the Bohr magneton, this gives:
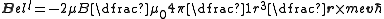 .
.Recognizing that mev is the electron momentum, p, and that r×p/ħ is the orbital angular momentum
Angular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
in units of ħ, l, we can write:
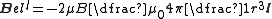 .
.For a many electron atom this expression is generally written in terms of the total orbital angular momentum,
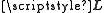 , by summing over the electrons and using the projection operator,
, by summing over the electrons and using the projection operator, 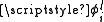 , where
, where 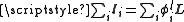 . For states with a well defined projection of the orbital angular momentum, Lz, we can write
. For states with a well defined projection of the orbital angular momentum, Lz, we can write 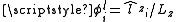 , giving:
, giving: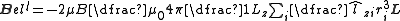 .
.The electron spin angular momentum is a fundamentally different property that is intrinsic to the particle and therefore does not depend on the motion of the electron. Nonetheless it is angular momentum and any angular momentum associated with a charged particle results in a magnetic dipole moment, which is the source of a magnetic field. An electron with spin angular momentum, s, has a magnetic moment, μs, given by:
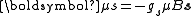 ,
,where gs is the electron spin g-factor and the negative sign is because the electron is negatively charged (consider that negatively and positively charged particles with identical mass, travelling on equivalent paths, would have the same angular momentum, but would result in currents in the opposite direction).
The magnetic field of a dipole moment, μs, is given by:
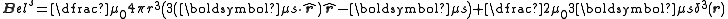 .
.The complete magnetic dipole contribution to the hyperfine Hamiltonian is thus given by:
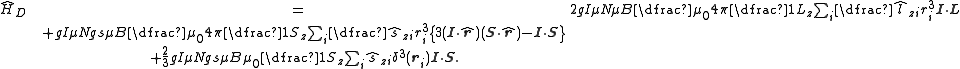
The first term gives the energy of the nuclear dipole in the field due to the electronic orbital angular momentum. The second term gives the energy of the "finite distance" interaction of the nuclear dipole with the field due to the electron spin magnetic moments. The final term, often known as the "Fermi contact
Fermi contact interaction
The Fermi contact interaction is the magnetic interaction between an electron and an atomic nucleus when the electron is inside that nucleus. It is of magnitude...
" term relates to the direct interaction of the nuclear dipole with the spin dipoles and is only non-zero for states with a finite electron spin density at the position of the nucleus (those with unpaired electrons in s-subshells). It has been argued that one may get a different expression when taking into account the detailed nuclear dipole moment distribution.
For states with l ≠ 0 this can be expressed in the form
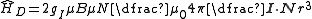 ,
,where
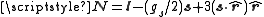 .
.If hyperfine structure is small compared with the fine structure (sometimes called IJ-coupling by analogy with LS-coupling), I and J are good quantum number
Quantum number
Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. Perhaps the most peculiar aspect of quantum mechanics is the quantization of observable quantities. This is distinguished from classical mechanics where the values can range continuously...
s and matrix elements of
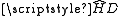 can be approximated as diagonal in I and J. In this case (generally true for light elements), we can project N onto J (where J = L + S is the total electronic angular momentum) and we have:
can be approximated as diagonal in I and J. In this case (generally true for light elements), we can project N onto J (where J = L + S is the total electronic angular momentum) and we have: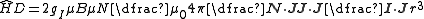 .
.This is commonly written as
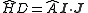 ,
,with
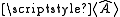 determined by experiment. Since I.J = ½{F.F - I.I - J.J} (where F = I + J is the total angular momentum), this gives an energy of
determined by experiment. Since I.J = ½{F.F - I.I - J.J} (where F = I + J is the total angular momentum), this gives an energy of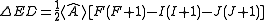 .
.In this case the hyperfine interaction satisfies the Lande interval rule
Landé interval rule
In atomic physics, the Landé interval rule states that if the spin-orbit interactions of an electron are weak, the energy levels of each are split. Subsequently, each have a different angular momentum...
.
Electric quadrupole
Atomic nuclei with spin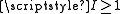 have an electric quadrupole moment. In the general case this is represented by a rank-2 tensor
have an electric quadrupole moment. In the general case this is represented by a rank-2 tensorTensor
Tensors are geometric objects that describe linear relations between vectors, scalars, and other tensors. Elementary examples include the dot product, the cross product, and linear maps. Vectors and scalars themselves are also tensors. A tensor can be represented as a multi-dimensional array of...
,
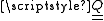 , with components given by:
, with components given by: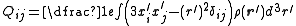 ,
,where i and j are the tensor indices running from 1 to 3, xi and xj are the spatial variables x, y and z depending on the values of i and j respectively, δij is the Kronecker delta and ρ(r) is the charge density. Being a 3-dimensional rank-2 tensor, the quadrupole moment has 32 = 9 components. From the definition of the components it is clear that the quadrupole tensor is a symmetric matrix (Qij = Qji) that is also traceless (ΣiQii = 0), giving only five components in the irreducible representation. Expressed using the notation of irreducible spherical tensors we have:
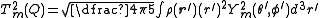 .
.The energy associated with an electric quadrupole moment in an electric field depends not on the field strength, but on the electric field gradient, confusingly labelled
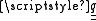 , another rank-2 tensor given by the outer product
, another rank-2 tensor given by the outer productOuter product
In linear algebra, the outer product typically refers to the tensor product of two vectors. The result of applying the outer product to a pair of vectors is a matrix...
of the del operator with the electric field vector:
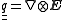 ,
,with components given by:
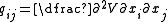 .
.Again it is clear this is a symmetric matrix and, because the source of the electric field at the nucleus is a charge distribution entirely outside the nucleus, this can be expressed as a 5-component spherical tensor,
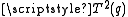 , with:
, with: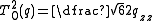
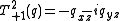
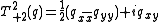 ,
,where:
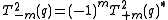 .
.The quadrupolar term in the Hamiltonian is thus given by:
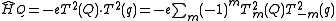 .
.A typical atomic nucleus closely approximates cylindrical symmetry and therefore all off-diagonal elements are close to zero. For this reason the nuclear electric quadrupole moment is often represented by Qzz.
Molecular hyperfine structure
The molecular hyperfine Hamiltonian includes those terms already derived for the atomic case with a magnetic dipole term for each nucleus with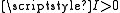 and an electric quadrupole term for each nucleus with
and an electric quadrupole term for each nucleus with 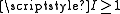 . The magnetic dipole terms were first derived for diatomic molecules by Frosch and Foley and the resulting hyperfine parameters are often called the Frosch and Foley parameters.
. The magnetic dipole terms were first derived for diatomic molecules by Frosch and Foley and the resulting hyperfine parameters are often called the Frosch and Foley parameters.In addition to the effects described above there are a number of effects specific to the molecular case.
Direct nuclear spin-spin
Each nucleus with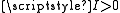 has a non-zero magnetic moment that is both the source of a magnetic field and has an associated energy due to the presence of the combined field of all of the other nuclear magnetic moments. A summation over each magnetic moment dotted with the field due to each other magnetic moment gives the direct nuclear spin-spin term in the hyperfine Hamiltonian,
has a non-zero magnetic moment that is both the source of a magnetic field and has an associated energy due to the presence of the combined field of all of the other nuclear magnetic moments. A summation over each magnetic moment dotted with the field due to each other magnetic moment gives the direct nuclear spin-spin term in the hyperfine Hamiltonian, 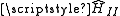 .
.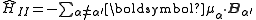 ,
,where α and α‘ are indices representing the nucleus contributing to the energy and the nucleus that is the source of the field respectively. Substituting in the expressions for the dipole moment in terms of the nuclear angular momentum and the magnetic field of a dipole, both given above, we have:
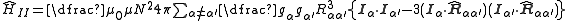 .
.Nuclear spin-rotation
The nuclear magnetic moments in a molecule exist in a magnetic field due to the angular momentum, T (R is the internuclear displacement vector), associated with the bulk rotation of the molecule.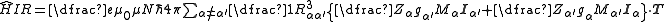
Measurements
Hyperfine interactions can be measured, among other ways, in atomic and molecular spectra and in electron paramagnetic resonanceElectron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
spectra of free radicals and transition-metal ions
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
.
Astrophysics
As the hyperfine splitting is very small, the transition frequencies usually are not optical, but in the range of radio- or microwave frequencies.Hyperfine structure gives the 21 cm line observed in H I region
H I region
An H I region is an interstellar cloud composed of neutral atomic hydrogen , in addition to the local abundance of helium and other elements. These regions are non-luminous, save for emission of the 21-cm region spectral line. This line has a very low transition probability, so requires large...
s in interstellar medium
Interstellar medium
In astronomy, the interstellar medium is the matter that exists in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, dust, and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space...
.
Carl Sagan
Carl Sagan
Carl Edward Sagan was an American astronomer, astrophysicist, cosmologist, author, science popularizer and science communicator in astronomy and natural sciences. He published more than 600 scientific papers and articles and was author, co-author or editor of more than 20 books...
and Frank Drake
Frank Drake
Frank Donald Drake PhD is an American astronomer and astrophysicist. He is most notable as one of the pioneers in the search for extraterrestrial intelligence, including the founding of SETI, mounting the first observational attempts at detecting extraterrestrial communications in 1961 in Project...
considered the hyperfine transition of hydrogen to be a sufficiently universal phenomenon so as to be used as a base unit of time and length on the Pioneer plaque
Pioneer plaque
The Pioneer plaques are a pair of gold-anodized aluminium plaques which were placed on board the 1972 Pioneer 10 and 1973 Pioneer 11 spacecraft, featuring a pictorial message, in case either Pioneer 10 or 11 are intercepted by extraterrestrial life...
and later Voyager Golden Record
Voyager Golden Record
The Voyager Golden Records are phonograph records which were included aboard both Voyager spacecraft, which were launched in 1977. They contain sounds and images selected to portray the diversity of life and culture on Earth, and are intended for any intelligent extraterrestrial life form, or for...
.
In radio astronomy
Radio astronomy
Radio astronomy is a subfield of astronomy that studies celestial objects at radio frequencies. The initial detection of radio waves from an astronomical object was made in the 1930s, when Karl Jansky observed radiation coming from the Milky Way. Subsequent observations have identified a number of...
, heterodyne receiver
Superheterodyne receiver
In electronics, a superheterodyne receiver uses frequency mixing or heterodyning to convert a received signal to a fixed intermediate frequency, which can be more conveniently processed than the original radio carrier frequency...
s are widely used in detection of the electromagnetic signals from celestial objects. The separations among various components of a hyperfine structure are usually small enough to fit into the receiver's IF
Intermediate frequency
In communications and electronic engineering, an intermediate frequency is a frequency to which a carrier frequency is shifted as an intermediate step in transmission or reception. The intermediate frequency is created by mixing the carrier signal with a local oscillator signal in a process called...
band. Because optical depth
Optical depth
Optical depth, or optical thickness, is a measure of transparency. Optical depth is defined by the negative logarithm of the fraction of radiation that is not scattered or absorbed on a path...
varies with frequency, strength ratios among the hyperfine components differ from that of their intrinsic intensities. From this we can derive the object's physical parameters.
Nuclear technology
The AVLISAVLIS
AVLIS Is an acronym which stands for atomic vapor laser isotope separation and is a method by which specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions....
process uses the hyperfine splitting between optical transitions in uranium-235 and uranium-238 to selectively photo-ionize only the uranium-235 atoms and then separate the ionized particles from the non-ionized ones. Precisely tuned dye laser
Dye laser
A dye laser is a laser which uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths. The wide bandwidth makes them particularly suitable for tunable lasers and...
s are used as the sources of the necessary exact wavelength radiation.
Use in defining the SI second and meter
The hyperfine structure transition can be used to make a microwaveMicrowave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
notch filter with very high stability, repeatability and Q factor
Q factor
In physics and engineering the quality factor or Q factor is a dimensionless parameter that describes how under-damped an oscillator or resonator is, or equivalently, characterizes a resonator's bandwidth relative to its center frequency....
, which can thus be used as a basis for very precise atomic clock
Atomic clock
An atomic clock is a clock that uses an electronic transition frequency in the microwave, optical, or ultraviolet region of the electromagnetic spectrum of atoms as a frequency standard for its timekeeping element...
s. Typically, the hyperfine structure transition frequency of a particular isotope of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
or rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
atoms is used as a basis for these clocks.
Due to the accuracy of hyperfine structure transition-based atomic clocks, they are now used as the basis for the definition of the second. One second
Second
The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock....
is now defined to be exactly 9,192,631,770 cycles of the hyperfine structure transition frequency of caesium-133 atoms.
Since 1983, the meter is defined by declaring the speed of light in a vacuum to be exactly 299,792,458 metres per second. Thus:
The metre is the length of the path travelled by light in vacuum during a time interval of 1/299,792,458 of a second.
Precision tests of quantum electrodynamics
The hyperfine splitting in hydrogen and in muoniumMuonium
Muonium is an exotic atom made up of an antimuon and an electron, which was discovered in 1960 and is given the chemical symbol . During the muon's lifetime, muonium can enter into compounds such as muonium chloride or sodium muonide . Due to the mass difference between the antimuon and the...
have been used to measure the value of the fine structure constant α. Comparison with measurements of α in other physical systems provides a stringent test of QED
Precision tests of QED
Quantum electrodynamics , a relativistic quantum field theory of electrodynamics, is among the most stringently tested theories in physics....
.
Qubit in ion-trap quantum computing
The hyperfine states of a trapped ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
are commonly used for storing qubit
Qubit
In quantum computing, a qubit or quantum bit is a unit of quantum information—the quantum analogue of the classical bit—with additional dimensions associated to the quantum properties of a physical atom....
s in ion-trap quantum computing. They have the advantage of having very long lifetimes, experimentally exceeding ~10 min (compared to ~1 s for metastable electronic levels).
The frequency associated with the states' energy separation is in the microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
region, making it possible to drive hyperfine transitions using microwave radiation. However, at present no emitter is available that can be focused to address a particular ion from a sequence. Instead, a pair of laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
pulses can be used to drive the transition, by having their frequency difference (detuning) equal to the required transition's frequency. This is essentially a stimulated Raman transition.
External links
- Nuclear Structure and Decay Data - IAEA Nuclear Magnetic and Electric Moments

