Hydrogen storage
Encyclopedia
Hydrogen storage describes the methods for storing H2 for subsequent use. The methods span many approaches, including high pressures, cryogenics, and chemical compounds that reversibly release H2 upon heating. Hydrogen storage is a topical goal in the development of a hydrogen economy
.
Most research into hydrogen storage is focused on storing hydrogen as a lightweight, compact energy carrier
for mobile applications
.
Liquid hydrogen
or slush hydrogen
may be used, as in the Space Shuttle
. However liquid hydrogen requires cryogenic storage and boils around 20.268 K
(–252.882 °C or -423.188 °F). Hence, its liquefaction
imposes a large energy loss (as energy is needed to cool it down to that temperature). The tanks must also be well insulated to prevent boil off
. Insulation by design for liquid hydrogen tanks is adding costs for this method. Liquid hydrogen has less energy density
by volume than hydrocarbon
fuels such as gasoline
by approximately a factor of four. This highlights the density problem for pure hydrogen: there is actually about 64% more hydrogen in a liter of gasoline (116 grams hydrogen) than there is in a liter of pure liquid hydrogen (71 grams hydrogen). The carbon in the gasoline also contributes to the energy of combustion.
Compressed hydrogen
, in comparison, is quite different to store. Hydrogen gas has good energy density by weight, but poor energy density by volume versus hydrocarbons, hence it requires a larger tank to store. A large hydrogen tank
will be heavier than the small hydrocarbon tank used to store the same amount of energy, all other factors remaining equal. Increasing gas pressure would improve the energy density by volume, making for smaller, but not lighter container tanks (see hydrogen tank
). Compressed hydrogen will require 2.1% of the energy content to power the compressor. Higher compression without energy recovery
will mean more energy lost to the compression step. Compressed hydrogen storage can exhibit very low permeation.
Some researchers have investigated underground hydrogen storage
to provide grid energy storage
for intermittent energy sources, like wind power
.
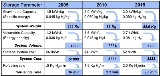
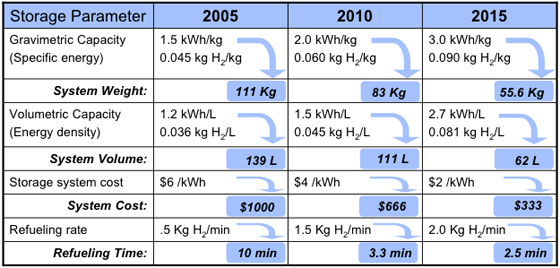 Targets were set by the FreedomCAR
Targets were set by the FreedomCAR
Partnership in January 2002 between the United States Council for Automotive Research (USCAR) and U.S. DOE
(Targets assume a 5-kg H2 storage system). The 2005 targets were not reached in 2005. The targets were revised in 2009 to reflect new data on system efficiencies obtained from fleets of test cars. The ultimate goal for volumetric storage is still above the theoretical density of liquid hydrogen.
It is important to note that these targets are for the hydrogen storage system, not the hydrogen storage material. System densities are often around half those of the working material, thus while a material may store 6 wt% H2, a working system using that material may only achieve 3 wt% when the weight of tanks, temperature and pressure control equipment, etc., is considered.
In 2010, only two storage technologies were identified as being susceptible to meet DOE targets: MOF-177 exceeds 2010 target for volumetric capacity, while cryo-compressed H2 exceeds more restrictive 2015 targets for both gravimetric and volumetric capacity (see slide 6 in ).
.
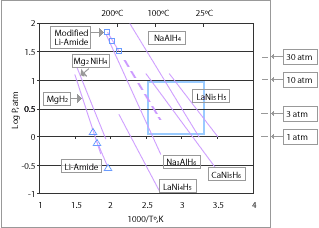 Metal hydrides, such as MgH2
Metal hydrides, such as MgH2
, NaAlH4
, LiAlH4, LiH
, LaNi5H6, and TiFeH2, with varying degrees of efficiency, can be used as a storage medium for hydrogen, often reversibly. Some are easy-to-fuel liquids at ambient temperature and pressure, others are solids which could be turned into pellets. These materials have good energy density by volume, although their energy density by weight is often worse than the leading hydrocarbon
fuels.
Most metal hydrides bind with hydrogen very strongly. As a result high temperatures around 120 °C (248 °F) - 200 °C (392 °F) are required to release their hydrogen content. This energy cost can be reduced by using alloys which consists of a strong hydride former and a weak one such as in LiNH2
, NaBH4
and LiBH4
. These are able to form weaker bonds, thereby requiring less input to release stored hydrogen. However if the interaction is too weak, the pressure needed for rehydriding is high, thereby eliminating any energy savings. The target for onboard hydrogen fuel systems is roughly <100 °C for release and <700 bar for recharge (20-60 kJ/mol H2 ).
Currently the only hydrides which are capable of achieving the 9 wt. % gravimetric goal for 2015 (see chart above) are limited to lithium, boron and aluminum based compounds; at least one of the first-row elements or Al must be added. Research is being done to determine new compounds which can be used to meet these requirements.
Proposed hydrides for use in a hydrogen economy include simple hydrides of magnesium
or transition metal
s and complex metal hydride
s, typically containing sodium
, lithium
, or calcium
and aluminium
or boron
. Hydrides chosen for storage applications provide low reactivity (high safety) and high hydrogen storage densities. Leading candidates are Lithium hydride
, sodium borohydride
, lithium aluminium hydride
and ammonia borane
. A French company McPhy Energy http://www.mcphy.com is developing the first industrial product, based on Magnesium Hydrate, already sold to some major clients such as Iwatani and ENEL.
New Scientist
stated that Arizona State University
is investigating using a borohydride solution to store hydrogen, which is released when the solution flows over a catalyst made of ruthenium.
Carbohydrates (Polymeric C6H10O5) releases H2 in an bioreformer mediated by the enzyme cocktail—cell-free synthetic pathway biotransformation. Carbohydrate provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a solid power. Carbohydrate is the most abundant renewable bioresource in the world.
In May 2007 biochemical engineers from the Virginia Polytechnic Institute and State University
and biologists and chemists from the Oak Ridge National Laboratory announced a method of producing high-yield pure hydrogen from starch and water. In 2009, they demonstrated to produce nearly 12 moles of hydrogen per glucose unit from cellulosic materials and water. Thanks to complete conversion and modest reaction conditions, they propose to use carbohydrate as a high energy density hydrogen carrier with a density of 14.8 wt %.
An alternative to hydrides is to use regular hydrocarbon
fuels as the hydrogen carrier. Then a small hydrogen reformer would extract the hydrogen as needed by the fuel cell
. However, these reformers are slow to react to changes in demand and add a large incremental cost to the vehicle powertrain.
Direct methanol fuel cells do not require a reformer, but provide a lower energy density compared to conventional fuel cells, although this could be counter balanced with the much better energy densities of ethanol
and methanol
over hydrogen. Alcohol fuel
is a renewable resource
.
Solid-oxide fuel cells can operate on light hydrocarbons such as propane
and methane
without a reformer, or can run on higher hydrocarbons with only partial reforming, but the high temperature and slow startup time of these fuel cells are problematic for automotive applications.
Ammonia
(NH3) releases H2 in an appropriate catalytic reformer. Ammonia provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a liquid at room temperature and pressure when mixed with water. Ammonia is the second most commonly produced chemical in the world and a large infrastructure for making, transporting, and distributing ammonia exists. Ammonia can be reformed to produce hydrogen with no harmful waste, or can mix with existing fuels and under the right conditions burn efficiently. Pure ammonia burns poorly at the atmospheric pressures found in natural gas fired water heaters and stoves. Under compression in an automobile engine it is a suitable fuel for slightly modified gasoline engines. Ammonia is a toxic gas at normal temperature and pressure and has a potent odor.
In September 2005 chemists from the Technical University of Denmark
announced a method of storing hydrogen in the form of ammonia
saturated into a salt tablet. They claim it will be an inexpensive and safe storage method.
Prior to 1980, several compounds were investigated for hydrogen storage including complex borohydrides, or aluminohydrides, and ammonium salts. These hydrides have an upper theoretical hydrogen yield limited to about 8.5% by weight. Amongst the compounds that contain only B, N, and H (both positive and negative ions), representative examples include: amine boranes, boron hydride ammoniates, hydrazine-borane complexes, and ammonium octahydrotriborates or tetrahydroborates. Of these, amine boranes (and especially ammonia borane
) have been extensively investigated as hydrogen carriers. During the 1970s and 1980s, the U.S. Army and Navy funded efforts aimed at developing hydrogen/deuterium gas-generating compounds for use in the HF/DF and HCl chemical laser
s, and gas dynamic lasers. Earlier hydrogen gas-generating formulations used amine boranes and their derivatives. Ignition of the amine borane(s) forms boron nitride
(BN) and hydrogen gas. In addition to ammonia borane
(H3BNH3), other gas-generators include diborane diammoniate, H2B(NH3)2BH4.
In 2006 researchers of EPFL, Switzerland, reported the use of formic acid
as a hydrogen storage material. Carbon monoxide free hydrogen has been generated in a very wide pressure range (1-600 bar). A homogeneous catalytic system based on water soluble ruthenium catalysts selectively decompose HCOOH into H2 and CO2 in aqueous solution. This catalytic system overcomes the limitations of other catalysts (e.g. poor stability, limited catalytic lifetimes, formation of CO) for the decomposition of formic acid making it a viable hydrogen storage material. And the co-product of this decomposition, carbon dioxide, can be used as hydrogen vector by hydrogenating it back to formic acid in a second step. The catalytic hydrogenation of CO2 has long been studied and efficient procedures have been developed. Formic acid contains 53 g L−1 hydrogen at room temperature and atmospheric pressure. By weight, pure formic acid stores 4.3 wt% hydrogen. Pure formic acid is a liquid with a flash point 69 °C (cf. gasoline -40 °C, ethanol 13 °C). 85% formic acid is not inflammable.
In 2007 Dupont J. and others reported hydrogen-storage materials based on imidazolium ionic liquids. Simple alkyl(aryl)-3-methylimidazolium N-bis(trifluoromethanesulfonyl)imidate salts that possess very low vapour pressure, high density, and thermal stability and are not inflammable can add reversibly 6-12 hydrogen atoms in the presence of classical Pd/C or Ir0 nanoparticle catalysts and can be used as alternative materials for on-board hydrogen-storage devices. These salts can hold up to 30 g L−1 of hydrogen at atmospheric pressure.
In 2006 researchers of University of Windsor
reported on reversible hydrogen storage in a non-metal phosphonium borate frustrated Lewis pair
:
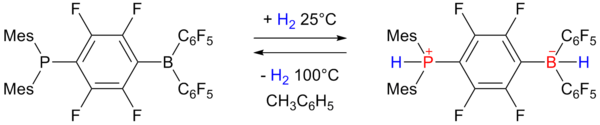 The phosphino-borane on the left accepts one equivalent of hydrogen at one atmosphere and 25 °C and expels it again by heating to 100 °C. The storage capacity is 0.25 wt% still rather below the 6 to 9 wt% required for practical use.
The phosphino-borane on the left accepts one equivalent of hydrogen at one atmosphere and 25 °C and expels it again by heating to 100 °C. The storage capacity is 0.25 wt% still rather below the 6 to 9 wt% required for practical use.
Research has proven that graphene
can store hydrogen efficiently. After taking up hydrogen, the substance becomes graphane
. After tests, conducted by dr André Geim at the University of Manchester
, it was shown that not only can graphene store hydrogen easily, it can also release the hydrogen again, after heating to 450 °C.
Cryo-compressed storage of hydrogen is the only technology that meets 2015 DOE targets for volumetric and gravimetric efficiency (see "CcH2" on slide 6 in ).
Furthermore, another study has shown that cryo-compressed exhibits interesting cost advantages : ownership cost ($ per mile) and storage system cost ($ per vehicle) are actually the lowest when compared to any other technology (see third row in slide 13 of ). For example, a cryo-compressed H2 system would cost $0.12 per mile (including cost of fuel and every associated other cost), while conventional gasoline vehicles cost between $0.05 and $0.07 per mile.
Alike liquid storage, cryo-compressed uses very cold H2 (20.3 K and slightly above) in order to reach a high energy density. However, the main difference is that, when the hydrogen would warm-up due to heat transfer with the environment ("boil off"), the tank is allowed to go to pressures much higher (up to 350 bars versus a couple of bars for liquid storage). As a consequence, it takes more time before the hydrogen has to vent, and in most driving situations, enough hydrogen is used by the car to keep the pressure well below the venting limit.
Consequently, it has been demonstrated that a high driving range could be achieved with a cryo-compressed tank : more than 650 miles (1,046.1 km) were driven with a full tank mounted on a H2 engine Prius. Research is still on its way in order to study and demonstrate the full potential of the technology.
As of 2010, the BMW Group has started a thorough component and system level validation of cryo-compressed vehicle storage on its way to a commercial product.
 Hydrogen carriers based on nanostructured carbon (such as carbon buckyballs
Hydrogen carriers based on nanostructured carbon (such as carbon buckyballs
and nanotubes
) have been proposed. Despite initial claims of greater than 50 wt% hydrogen storage, it has generally come to be accepted that less than 1 wt% is practical.
Metal-organic framework
s represent another class of synthetic porous materials that store hydrogen and energy at the molecular level. MOFs are highly crystalline inorganic-organic hybrid structures that contain metal clusters or ions (secondary building units) as nodes and organic ligands as linkers. When guest molecules (solvent) occupying the pores are removed during solvent exchange and heating under vacuum, porous structure of MOFs can be achieved without destabilizing the frame and hydrogen molecules will be adsorbed onto the surface of the pores by physisorption. Compared to traditional zeolites and porous carbon materials, MOFs have very high number of pores and surface area which allow higher hydrogen uptake in a given volume. Thus, research interests on hydrogen storage in MOFs have been growing since 2003 when the first MOF-based hydrogen storage was introduced. Since there are infinite geometric and chemical variations of MOFs based on different combinations of SBUs and linkers, many researches explore what combination will provide the maximum hydrogen uptake by varying materials of metal ions and linkers.
In 2006, chemists at UCLA and the University of Michigan
have achieved hydrogen storage concentrations of up to 7.5 wt% in MOF-74 at a low temperature of 77K
. In 2009, researchers at University of Nottingham
reached 10 wt% at 77 bar (1,117 psi) and 77 K with MOF NOTT-112. Most articles about hydrogen storage in MOFs report hydrogen uptake capacity at a temperature of 77K and a pressure of 1 bar because such condition is commonly available and the binding energy between hydrogen and MOF is large compare to the thermal vibration energy which will allow high hydrogen uptake capacity. Varying several factors such as surface area, pore size, catenation, ligand structure, spillover, and sample purity can result different amount of hydrogen uptake in MOFs.
H2 caged
in a clathrate hydrate
was first reported in 2002, but requires very high pressures to be stable. In 2004, researchers from Delft University of Technology
and Colorado School of Mines
showed solid H2-containing hydrates could be formed at ambient temperature and 10s of bar
by adding small amounts of promoting substances such as THF
. These clathrates have a theoretical maximum hydrogen densities of around 5 wt% and 40 kg/m3.
In 2006, a team of Korea
n researchers led by Professor Jisoon Ihm at Department of Physics and Astronomy of Seoul National University
proposed a new material with the hydrogen storage efficiency of 7.6 percent based on first-principles electronic structure calculations for hydrogen binding to metal-decorated polymers of many different kinds. According to these researchers, hydrogen can be stored in a solid material at ambient temperatures and pressures by attaching a titanium
atom to a polyacetylene
. http://english.hani.co.kr/arti/english_edition/e_business/146855.htmlhttp://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=PRLTAO000097000005056104000001&idtype=cvips&gifs=Yes
A team of Russian, Israeli and German scientists have collaboratively developed an innovative technology based on glass capillary arrays for the safe infusion, storage and controlled release of hydrogen in mobile applications http://dx.doi.org/10.1016/j.enconman.2006.11.017http://dx.doi.org/10.1016/j.ijhydene.2009.10.011. The C.En http://www.cenh2go.com technology has achieved the US Department of Energy (DOE) 2010 targets for on-board hydrogen storage systems.
Hollow glass microspheres (HGM) can be utilized for controlled storage and release of hydrogen.
Keratine, a compound found in bird feathers, has been found to be useful to increase the interior surface (and thus the hydrogen storage capacity) of tanks. The research stated that the use of carbonized chicken feather fibres would result in far lower manufacturing costs than other common hydrogen tanks on the market. The research was conducted by Richard Wool and Erman Senoz.
is the practice of hydrogen storage in underground cave
rns, salt dome
s and depleted oil and gas fields. Large quantities of gaseous hydrogen have been stored in underground caverns by ICI
for many years without any difficulties. The storage of large quantities of hydrogen underground can function as grid energy storage
which is essential for the hydrogen economy
.
Hydrogen economy
The hydrogen economy is a proposed system of delivering energy using hydrogen. The term hydrogen economy was coined by John Bockris during a talk he gave in 1970 at General Motors Technical Center....
.
Most research into hydrogen storage is focused on storing hydrogen as a lightweight, compact energy carrier
Energy carrier
According to ISO 13600, an energy carrier is either a substance or a phenomenon that can be used to produce mechanical work or heat or to operate chemical or physical processes....
for mobile applications
Hydrogen vehicle
A hydrogen vehicle is a vehicle that uses hydrogen as its onboard fuel for motive power. Hydrogen vehicles include hydrogen fueled space rockets, as well as automobiles and other transportation vehicles...
.
Liquid hydrogen
Liquid hydrogen
Liquid hydrogen is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.To exist as a liquid, H2 must be pressurized above and cooled below hydrogen's Critical point. However, for hydrogen to be in a full liquid state without boiling off, it needs to be...
or slush hydrogen
Slush hydrogen
Slush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is formed by bringing liquid hydrogen down to nearly the melting point that increases density by 16–20% as compared to liquid hydrogen...
may be used, as in the Space Shuttle
Space Shuttle
The Space Shuttle was a manned orbital rocket and spacecraft system operated by NASA on 135 missions from 1981 to 2011. The system combined rocket launch, orbital spacecraft, and re-entry spaceplane with modular add-ons...
. However liquid hydrogen requires cryogenic storage and boils around 20.268 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
(–252.882 °C or -423.188 °F). Hence, its liquefaction
Liquefaction
Liquefaction may refer to:* Liquefaction, the general process of becoming liquid* Soil liquefaction, the process by which sediments become suspended* Liquefaction of gases in physics, chemistry, and thermal engineering* Liquefactive necrosis in pathology...
imposes a large energy loss (as energy is needed to cool it down to that temperature). The tanks must also be well insulated to prevent boil off
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
. Insulation by design for liquid hydrogen tanks is adding costs for this method. Liquid hydrogen has less energy density
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
by volume than hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
fuels such as gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
by approximately a factor of four. This highlights the density problem for pure hydrogen: there is actually about 64% more hydrogen in a liter of gasoline (116 grams hydrogen) than there is in a liter of pure liquid hydrogen (71 grams hydrogen). The carbon in the gasoline also contributes to the energy of combustion.
Compressed hydrogen
Compressed hydrogen
Compressed hydrogen is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar and 700 bar is used for mobile hydrogen storage in hydrogen vehicles...
, in comparison, is quite different to store. Hydrogen gas has good energy density by weight, but poor energy density by volume versus hydrocarbons, hence it requires a larger tank to store. A large hydrogen tank
Hydrogen tank
A Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 Bar were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the HydroGen4.At the hydrogen station Hamburg...
will be heavier than the small hydrocarbon tank used to store the same amount of energy, all other factors remaining equal. Increasing gas pressure would improve the energy density by volume, making for smaller, but not lighter container tanks (see hydrogen tank
Hydrogen tank
A Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 Bar were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the HydroGen4.At the hydrogen station Hamburg...
). Compressed hydrogen will require 2.1% of the energy content to power the compressor. Higher compression without energy recovery
Energy recovery
Energy recovery includes any technique or method of minimizing the input of energy to an overall system by the exchange of energy from one sub-system of the overall system with another...
will mean more energy lost to the compression step. Compressed hydrogen storage can exhibit very low permeation.
Some researchers have investigated underground hydrogen storage
Underground hydrogen storage
Underground hydrogen storage is the practice of hydrogen storage in underground caverns, salt domes and depleted oil/gas fields. Large quantities of gaseous hydrogen have been stored in underground caverns by ICI for many years without any difficulties...
to provide grid energy storage
Grid energy storage
Grid energy storage refers to the methods used to store electricity on a large scale within an electrical power grid. Electrical energy is stored during times when production exceeds consumption and the stores are used at times when consumption exceeds production...
for intermittent energy sources, like wind power
Wind power
Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships....
.
Onboard hydrogen storage

FreedomCAR
The FreedomCAR and Vehicle Technologies is a U.S. national Office of Energy Efficiency and Renewable Energy program developing more energy efficient and environmentally friendly highway transportation technologies that will enable the U.S to use less petroleum...
Partnership in January 2002 between the United States Council for Automotive Research (USCAR) and U.S. DOE
United States Department of Energy
The United States Department of Energy is a Cabinet-level department of the United States government concerned with the United States' policies regarding energy and safety in handling nuclear material...
(Targets assume a 5-kg H2 storage system). The 2005 targets were not reached in 2005. The targets were revised in 2009 to reflect new data on system efficiencies obtained from fleets of test cars. The ultimate goal for volumetric storage is still above the theoretical density of liquid hydrogen.
It is important to note that these targets are for the hydrogen storage system, not the hydrogen storage material. System densities are often around half those of the working material, thus while a material may store 6 wt% H2, a working system using that material may only achieve 3 wt% when the weight of tanks, temperature and pressure control equipment, etc., is considered.
In 2010, only two storage technologies were identified as being susceptible to meet DOE targets: MOF-177 exceeds 2010 target for volumetric capacity, while cryo-compressed H2 exceeds more restrictive 2015 targets for both gravimetric and volumetric capacity (see slide 6 in ).
Compressed hydrogen
Compressed hydrogen is the gaseous state of the element hydrogen which is kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for in hydrogen vehicles. Car manufacturers have been developing this solution, such as Honda or Nissan.Liquid hydrogen
BMW has been working on liquid tank for cars, producing for example the BMW Hydrogen 7BMW Hydrogen 7
The BMW Hydrogen 7 is a limited production hydrogen vehicle built by German automobile manufacturer BMW. The car is based on BMW’s traditional gasoline powered 7-series line of vehicles, and more specifically the 760Li...
.
Proposals and research
Hydrogen storage technologies can be divided into physical storage, where hydrogen molecules are stored (including pure hydrogen storage via compression and liquefication), and chemical storage, where hydrides are stored.Metal hydrides

Magnesium hydride
Magnesium hydride is the chemical compound MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.- Preparation :...
, NaAlH4
Sodium aluminium hydride
Sodium aluminium hydride or sodium alanate is a chemical compound used as a reducing agent. It is similar to lithium aluminium hydride....
, LiAlH4, LiH
Lithium hydride
Lithium hydride is the inorganic compound with the formula LiH. It is a colorless solid, although commercial samples are gray. Characteristic of a salt-like, or ionic, hydride, it has a high melting point and is not soluble in any solvent with which it does not react...
, LaNi5H6, and TiFeH2, with varying degrees of efficiency, can be used as a storage medium for hydrogen, often reversibly. Some are easy-to-fuel liquids at ambient temperature and pressure, others are solids which could be turned into pellets. These materials have good energy density by volume, although their energy density by weight is often worse than the leading hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
fuels.
Most metal hydrides bind with hydrogen very strongly. As a result high temperatures around 120 °C (248 °F) - 200 °C (392 °F) are required to release their hydrogen content. This energy cost can be reduced by using alloys which consists of a strong hydride former and a weak one such as in LiNH2
Lithium amide
Lithium amide is an inorganic compound with the chemical formula Li+NH2-, i.e. it is composed of a lithium cation, and the conjugate base of ammonia. It is a white solid with a tetragonal crystal structure.-Lithium amides:...
, NaBH4
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
and LiBH4
Lithium borohydride
Lithium borohydride is a tetrahydroborate and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages of being highly soluble in ethers and being a stronger reducing agent but still safer to...
. These are able to form weaker bonds, thereby requiring less input to release stored hydrogen. However if the interaction is too weak, the pressure needed for rehydriding is high, thereby eliminating any energy savings. The target for onboard hydrogen fuel systems is roughly <100 °C for release and <700 bar for recharge (20-60 kJ/mol H2 ).
Currently the only hydrides which are capable of achieving the 9 wt. % gravimetric goal for 2015 (see chart above) are limited to lithium, boron and aluminum based compounds; at least one of the first-row elements or Al must be added. Research is being done to determine new compounds which can be used to meet these requirements.
Proposed hydrides for use in a hydrogen economy include simple hydrides of magnesium
Magnesium hydride
Magnesium hydride is the chemical compound MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.- Preparation :...
or transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s and complex metal hydride
Complex metal hydride
Complex metal hydrides are salts wherein the anions contain hydrides. In the older chemical literature as well as contemporary materials science textbooks, a "metal hydride" is assumed to be nonmolecular, i.e. three-dimensional lattices of atomic ions...
s, typically containing sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
, or calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
and aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
or boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
. Hydrides chosen for storage applications provide low reactivity (high safety) and high hydrogen storage densities. Leading candidates are Lithium hydride
Lithium hydride
Lithium hydride is the inorganic compound with the formula LiH. It is a colorless solid, although commercial samples are gray. Characteristic of a salt-like, or ionic, hydride, it has a high melting point and is not soluble in any solvent with which it does not react...
, sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
, lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
and ammonia borane
Ammonia borane
Ammonia borane is the chemical compound with the formula H3NBH3. The colourless solid is the simplest molecular boron-nitrogen-hydride compound and has attracted attention as a source of hydrogen fuel.-Synthesis:...
. A French company McPhy Energy http://www.mcphy.com is developing the first industrial product, based on Magnesium Hydrate, already sold to some major clients such as Iwatani and ENEL.
New Scientist
New Scientist
New Scientist is a weekly non-peer-reviewed English-language international science magazine, which since 1996 has also run a website, covering recent developments in science and technology for a general audience. Founded in 1956, it is published by Reed Business Information Ltd, a subsidiary of...
stated that Arizona State University
Arizona State University
Arizona State University is a public research university located in the Phoenix Metropolitan Area of the State of Arizona...
is investigating using a borohydride solution to store hydrogen, which is released when the solution flows over a catalyst made of ruthenium.
Carbohydrates
Carbohydrates (Polymeric C6H10O5) releases H2 in an bioreformer mediated by the enzyme cocktail—cell-free synthetic pathway biotransformation. Carbohydrate provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a solid power. Carbohydrate is the most abundant renewable bioresource in the world.
In May 2007 biochemical engineers from the Virginia Polytechnic Institute and State University
Virginia Polytechnic Institute and State University
Virginia Polytechnic Institute and State University, popularly known as Virginia Tech , is a public land-grant university with the main campus in Blacksburg, Virginia with other research and educational centers throughout the Commonwealth of Virginia, United States, and internationally.Founded in...
and biologists and chemists from the Oak Ridge National Laboratory announced a method of producing high-yield pure hydrogen from starch and water. In 2009, they demonstrated to produce nearly 12 moles of hydrogen per glucose unit from cellulosic materials and water. Thanks to complete conversion and modest reaction conditions, they propose to use carbohydrate as a high energy density hydrogen carrier with a density of 14.8 wt %.
Synthesized hydrocarbons
An alternative to hydrides is to use regular hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
fuels as the hydrogen carrier. Then a small hydrogen reformer would extract the hydrogen as needed by the fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
. However, these reformers are slow to react to changes in demand and add a large incremental cost to the vehicle powertrain.
Direct methanol fuel cells do not require a reformer, but provide a lower energy density compared to conventional fuel cells, although this could be counter balanced with the much better energy densities of ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
over hydrogen. Alcohol fuel
Alcohol fuel
Although fossil fuels have become the dominant energy resource for the modern world, alcohol has been used as a fuel throughout history. The first four aliphatic alcohols are of interest as fuels because they can be synthesized chemically or biologically, and they have characteristics which allow...
is a renewable resource
Renewable resource
A renewable resource is a natural resource with the ability of being replaced through biological or other natural processes and replenished with the passage of time...
.
Solid-oxide fuel cells can operate on light hydrocarbons such as propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
and methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
without a reformer, or can run on higher hydrocarbons with only partial reforming, but the high temperature and slow startup time of these fuel cells are problematic for automotive applications.
Ammonia
Ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(NH3) releases H2 in an appropriate catalytic reformer. Ammonia provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a liquid at room temperature and pressure when mixed with water. Ammonia is the second most commonly produced chemical in the world and a large infrastructure for making, transporting, and distributing ammonia exists. Ammonia can be reformed to produce hydrogen with no harmful waste, or can mix with existing fuels and under the right conditions burn efficiently. Pure ammonia burns poorly at the atmospheric pressures found in natural gas fired water heaters and stoves. Under compression in an automobile engine it is a suitable fuel for slightly modified gasoline engines. Ammonia is a toxic gas at normal temperature and pressure and has a potent odor.
In September 2005 chemists from the Technical University of Denmark
Technical University of Denmark
The Technical University of Denmark , often simply referred to as DTU, is a university just north of Copenhagen, Denmark. It was founded in 1829 at the initiative of Hans Christian Ørsted as Denmark's first polytechnic, and is today ranked among Europe's leading engineering institutions, and the...
announced a method of storing hydrogen in the form of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
saturated into a salt tablet. They claim it will be an inexpensive and safe storage method.
Amine borane complexes
Prior to 1980, several compounds were investigated for hydrogen storage including complex borohydrides, or aluminohydrides, and ammonium salts. These hydrides have an upper theoretical hydrogen yield limited to about 8.5% by weight. Amongst the compounds that contain only B, N, and H (both positive and negative ions), representative examples include: amine boranes, boron hydride ammoniates, hydrazine-borane complexes, and ammonium octahydrotriborates or tetrahydroborates. Of these, amine boranes (and especially ammonia borane
Ammonia borane
Ammonia borane is the chemical compound with the formula H3NBH3. The colourless solid is the simplest molecular boron-nitrogen-hydride compound and has attracted attention as a source of hydrogen fuel.-Synthesis:...
) have been extensively investigated as hydrogen carriers. During the 1970s and 1980s, the U.S. Army and Navy funded efforts aimed at developing hydrogen/deuterium gas-generating compounds for use in the HF/DF and HCl chemical laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
s, and gas dynamic lasers. Earlier hydrogen gas-generating formulations used amine boranes and their derivatives. Ignition of the amine borane(s) forms boron nitride
Boron nitride
Boron nitride is a chemical compound with chemical formula BN, consisting of equal numbers of boron and nitrogen atoms. BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms...
(BN) and hydrogen gas. In addition to ammonia borane
(H3BNH3), other gas-generators include diborane diammoniate, H2B(NH3)2BH4.
Formic acid
In 2006 researchers of EPFL, Switzerland, reported the use of formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
as a hydrogen storage material. Carbon monoxide free hydrogen has been generated in a very wide pressure range (1-600 bar). A homogeneous catalytic system based on water soluble ruthenium catalysts selectively decompose HCOOH into H2 and CO2 in aqueous solution. This catalytic system overcomes the limitations of other catalysts (e.g. poor stability, limited catalytic lifetimes, formation of CO) for the decomposition of formic acid making it a viable hydrogen storage material. And the co-product of this decomposition, carbon dioxide, can be used as hydrogen vector by hydrogenating it back to formic acid in a second step. The catalytic hydrogenation of CO2 has long been studied and efficient procedures have been developed. Formic acid contains 53 g L−1 hydrogen at room temperature and atmospheric pressure. By weight, pure formic acid stores 4.3 wt% hydrogen. Pure formic acid is a liquid with a flash point 69 °C (cf. gasoline -40 °C, ethanol 13 °C). 85% formic acid is not inflammable.
Imidazolium ionic liquids
In 2007 Dupont J. and others reported hydrogen-storage materials based on imidazolium ionic liquids. Simple alkyl(aryl)-3-methylimidazolium N-bis(trifluoromethanesulfonyl)imidate salts that possess very low vapour pressure, high density, and thermal stability and are not inflammable can add reversibly 6-12 hydrogen atoms in the presence of classical Pd/C or Ir0 nanoparticle catalysts and can be used as alternative materials for on-board hydrogen-storage devices. These salts can hold up to 30 g L−1 of hydrogen at atmospheric pressure.
Phosphonium borate
In 2006 researchers of University of Windsor
University of Windsor
The University of Windsor is a public comprehensive and research university in Windsor, Ontario, Canada. It is Canada's southernmost university. It has a student population of approximately 15,000 full-time and part-time undergraduate students and over 1000 graduate students...
reported on reversible hydrogen storage in a non-metal phosphonium borate frustrated Lewis pair
Frustrated Lewis pair
In chemistry, a frustrated Lewis pair is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form an adduct...
:
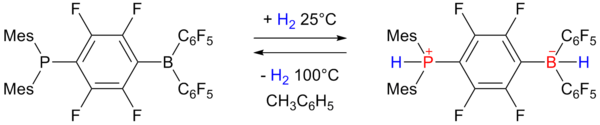
Carbonite substances
Research has proven that graphene
Graphene
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer...
can store hydrogen efficiently. After taking up hydrogen, the substance becomes graphane
Graphane
Graphane is a 2-dimensional polymer of carbon and hydrogen with the formula unit n where n is large. Graphane's carbon bonds are in sp3 configuration, as opposed to graphene's sp2 bond configuration, thus graphane is a 2-D analog of cubic diamond. Graphane is a form of hydrogenated graphene...
. After tests, conducted by dr André Geim at the University of Manchester
University of Manchester
The University of Manchester is a public research university located in Manchester, United Kingdom. It is a "red brick" university and a member of the Russell Group of research-intensive British universities and the N8 Group...
, it was shown that not only can graphene store hydrogen easily, it can also release the hydrogen again, after heating to 450 °C.
Cryo-compressed
Cryo-compressed storage of hydrogen is the only technology that meets 2015 DOE targets for volumetric and gravimetric efficiency (see "CcH2" on slide 6 in ).
Furthermore, another study has shown that cryo-compressed exhibits interesting cost advantages : ownership cost ($ per mile) and storage system cost ($ per vehicle) are actually the lowest when compared to any other technology (see third row in slide 13 of ). For example, a cryo-compressed H2 system would cost $0.12 per mile (including cost of fuel and every associated other cost), while conventional gasoline vehicles cost between $0.05 and $0.07 per mile.
Alike liquid storage, cryo-compressed uses very cold H2 (20.3 K and slightly above) in order to reach a high energy density. However, the main difference is that, when the hydrogen would warm-up due to heat transfer with the environment ("boil off"), the tank is allowed to go to pressures much higher (up to 350 bars versus a couple of bars for liquid storage). As a consequence, it takes more time before the hydrogen has to vent, and in most driving situations, enough hydrogen is used by the car to keep the pressure well below the venting limit.
Consequently, it has been demonstrated that a high driving range could be achieved with a cryo-compressed tank : more than 650 miles (1,046.1 km) were driven with a full tank mounted on a H2 engine Prius. Research is still on its way in order to study and demonstrate the full potential of the technology.
As of 2010, the BMW Group has started a thorough component and system level validation of cryo-compressed vehicle storage on its way to a commercial product.
Carbon nanotubes

Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
and nanotubes
Carbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
) have been proposed. Despite initial claims of greater than 50 wt% hydrogen storage, it has generally come to be accepted that less than 1 wt% is practical.
Metal-organic frameworks
Metal-organic framework
Metal-organic framework
Metal-Organic Frameworks are crystalline compounds consisting of metal ions or clusters coordinated to often rigid organic molecules to form one-, two-, or three-dimensional structures that can be porous. In some cases, the pores are stable to elimination of the guest molecules and can be used for...
s represent another class of synthetic porous materials that store hydrogen and energy at the molecular level. MOFs are highly crystalline inorganic-organic hybrid structures that contain metal clusters or ions (secondary building units) as nodes and organic ligands as linkers. When guest molecules (solvent) occupying the pores are removed during solvent exchange and heating under vacuum, porous structure of MOFs can be achieved without destabilizing the frame and hydrogen molecules will be adsorbed onto the surface of the pores by physisorption. Compared to traditional zeolites and porous carbon materials, MOFs have very high number of pores and surface area which allow higher hydrogen uptake in a given volume. Thus, research interests on hydrogen storage in MOFs have been growing since 2003 when the first MOF-based hydrogen storage was introduced. Since there are infinite geometric and chemical variations of MOFs based on different combinations of SBUs and linkers, many researches explore what combination will provide the maximum hydrogen uptake by varying materials of metal ions and linkers.
In 2006, chemists at UCLA and the University of Michigan
University of Michigan
The University of Michigan is a public research university located in Ann Arbor, Michigan in the United States. It is the state's oldest university and the flagship campus of the University of Michigan...
have achieved hydrogen storage concentrations of up to 7.5 wt% in MOF-74 at a low temperature of 77K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
. In 2009, researchers at University of Nottingham
University of Nottingham
The University of Nottingham is a public research university based in Nottingham, United Kingdom, with further campuses in Ningbo, China and Kuala Lumpur, Malaysia...
reached 10 wt% at 77 bar (1,117 psi) and 77 K with MOF NOTT-112. Most articles about hydrogen storage in MOFs report hydrogen uptake capacity at a temperature of 77K and a pressure of 1 bar because such condition is commonly available and the binding energy between hydrogen and MOF is large compare to the thermal vibration energy which will allow high hydrogen uptake capacity. Varying several factors such as surface area, pore size, catenation, ligand structure, spillover, and sample purity can result different amount of hydrogen uptake in MOFs.
Clathrate hydrates
H2 caged
Hydrogen clathrate
A hydrogen clathrate is a clathrate containing hydrogen in water ice. This substance is interesting due to its possible use to store hydrogen in a hydrogen economy. Also interesting is that multiple hydrogen molecules can occur at each cage site in the ice. The maximum ration of hydrogen to water...
in a clathrate hydrate
Clathrate hydrate
Clathrate hydrates are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded water molecules...
was first reported in 2002, but requires very high pressures to be stable. In 2004, researchers from Delft University of Technology
Delft University of Technology
Delft University of Technology , also known as TU Delft, is the largest and oldest Dutch public technical university, located in Delft, Netherlands...
and Colorado School of Mines
Colorado School of Mines
The Colorado School of Mines is a small public teaching and research university devoted to engineering and applied science, with special expertise in the development and stewardship of the Earth's natural resources. Located in Golden, Colorado, CSM was ranked 29th, in America among national...
showed solid H2-containing hydrates could be formed at ambient temperature and 10s of bar
Bar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
by adding small amounts of promoting substances such as THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
. These clathrates have a theoretical maximum hydrogen densities of around 5 wt% and 40 kg/m3.
Doped polymers
In 2006, a team of Korea
Korea
Korea ) is an East Asian geographic region that is currently divided into two separate sovereign states — North Korea and South Korea. Located on the Korean Peninsula, Korea is bordered by the People's Republic of China to the northwest, Russia to the northeast, and is separated from Japan to the...
n researchers led by Professor Jisoon Ihm at Department of Physics and Astronomy of Seoul National University
Seoul National University
Seoul National University , colloquially known in Korean as Seoul-dae , is a national research university in Seoul, Korea, ranked 24th in the world in publications in an analysis of data from the Science Citation Index, 7th in Asia and 42nd in the world by the 2011 QS World University Rankings...
proposed a new material with the hydrogen storage efficiency of 7.6 percent based on first-principles electronic structure calculations for hydrogen binding to metal-decorated polymers of many different kinds. According to these researchers, hydrogen can be stored in a solid material at ambient temperatures and pressures by attaching a titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
atom to a polyacetylene
Polyacetylene
Polyacetylene is an organic polymer with the repeat unit n. The high electrical conductivity discovered for these polymers beginning in the 1960's accelerated interest in the use of organic compounds in microelectronics...
. http://english.hani.co.kr/arti/english_edition/e_business/146855.htmlhttp://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=PRLTAO000097000005056104000001&idtype=cvips&gifs=Yes
Glass Capillary Arrays
A team of Russian, Israeli and German scientists have collaboratively developed an innovative technology based on glass capillary arrays for the safe infusion, storage and controlled release of hydrogen in mobile applications http://dx.doi.org/10.1016/j.enconman.2006.11.017http://dx.doi.org/10.1016/j.ijhydene.2009.10.011. The C.En http://www.cenh2go.com technology has achieved the US Department of Energy (DOE) 2010 targets for on-board hydrogen storage systems.
Glass microspheres
Hollow glass microspheres (HGM) can be utilized for controlled storage and release of hydrogen.
Keratine
Keratine, a compound found in bird feathers, has been found to be useful to increase the interior surface (and thus the hydrogen storage capacity) of tanks. The research stated that the use of carbonized chicken feather fibres would result in far lower manufacturing costs than other common hydrogen tanks on the market. The research was conducted by Richard Wool and Erman Senoz.
Stationary hydrogen storage
Unlike mobile applications, hydrogen density is not a huge problem for stationary applications. As for mobile applications, stationary applications can use established technology:- Compressed hydrogenCompressed hydrogenCompressed hydrogen is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar and 700 bar is used for mobile hydrogen storage in hydrogen vehicles...
(CGH2) in a hydrogen tankHydrogen tankA Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 Bar were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the HydroGen4.At the hydrogen station Hamburg... - Liquid hydrogenLiquid hydrogenLiquid hydrogen is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.To exist as a liquid, H2 must be pressurized above and cooled below hydrogen's Critical point. However, for hydrogen to be in a full liquid state without boiling off, it needs to be...
in a (LH2) cryogenic hydrogen tank - Slush hydrogenSlush hydrogenSlush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is formed by bringing liquid hydrogen down to nearly the melting point that increases density by 16–20% as compared to liquid hydrogen...
in a cryogenic hydrogen tank
Underground hydrogen storage
Underground hydrogen storageUnderground hydrogen storage
Underground hydrogen storage is the practice of hydrogen storage in underground caverns, salt domes and depleted oil/gas fields. Large quantities of gaseous hydrogen have been stored in underground caverns by ICI for many years without any difficulties...
is the practice of hydrogen storage in underground cave
Cave
A cave or cavern is a natural underground space large enough for a human to enter. The term applies to natural cavities some part of which is in total darkness. The word cave also includes smaller spaces like rock shelters, sea caves, and grottos.Speleology is the science of exploration and study...
rns, salt dome
Salt dome
A salt dome is a type of structural dome formed when a thick bed of evaporite minerals found at depth intrudes vertically into surrounding rock strata, forming a diapir....
s and depleted oil and gas fields. Large quantities of gaseous hydrogen have been stored in underground caverns by ICI
Imperial Chemical Industries
Imperial Chemical Industries was a British chemical company, taken over by AkzoNobel, a Dutch conglomerate, one of the largest chemical producers in the world. In its heyday, ICI was the largest manufacturing company in the British Empire, and commonly regarded as a "bellwether of the British...
for many years without any difficulties. The storage of large quantities of hydrogen underground can function as grid energy storage
Grid energy storage
Grid energy storage refers to the methods used to store electricity on a large scale within an electrical power grid. Electrical energy is stored during times when production exceeds consumption and the stores are used at times when consumption exceeds production...
which is essential for the hydrogen economy
Hydrogen economy
The hydrogen economy is a proposed system of delivering energy using hydrogen. The term hydrogen economy was coined by John Bockris during a talk he gave in 1970 at General Motors Technical Center....
.
See also
- Lithium borohydrideLithium borohydrideLithium borohydride is a tetrahydroborate and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages of being highly soluble in ethers and being a stronger reducing agent but still safer to...
- Cascade storage system
- Complex hydride
- Cryo-adsorptionCryo-adsorptionCryo-adsorption is a method used for hydrogen storage where gaseous hydrogen at cryogenic temperatures is physically adsorbed on porous material, mostly activated carbon. The achievable storage density is between liquid hydrogen storage systems and compressed hydrogen storage systems.-External...
- HydrogenographyHydrogenographyHydrogenography is a combinatorial method based on the observation of optical changes on the metal surface by hydrogen absorption. The method allows the examination of thousands of combinations of alloy samples in a single batch.-History:...
- Hydrogen tankHydrogen tankA Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 Bar were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the HydroGen4.At the hydrogen station Hamburg...
- Hydrogen molecular technologies
- Hydrogen energy plant in DenmarkHydrogen energy plant in DenmarkDenmark's first full-scale wind-Hydrogen energy plant and testing facility, the Lolland Hydrogen Community, began operation in May 2007. It is also the European Union's first full-scale Hydrogen Community Demonstration facility for residential Fuel Cell Combined Heat and Power .Located in the city...
- Tunable nanoporous carbonTunable nanoporous carbonUltracapacitors may have the potential to become key components for energy storage in the industrial market with the rising push for environmental technology. There are several different approaches to creating ultracapacitors, as detailed here, and tunable nanoporous carbon is a relatively new...
External links
- EU Storhy
- Nesshy
- Hycones
- United States Department of Energy Planned program activities for 2003-2010
- Ammonia Borane (NhxBHx)
- C.En
- Hyweb (1996)
- Research into metal-organic framework or Nano Cages http://www.nist.gov/public_affairs/techbeat/tb2005_120105.htm#cageshttp://www.ncnr.nist.gov/staff/taner/h2/
- Hydrogen Storage Technical Data

