Histidine kinase
Encyclopedia
Histidine Kinases are multifunctional, typically transmembrane, proteins of the transferase
class that play a role in signal transduction
across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase
, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors
for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell (extracellular
domain) that bind to hormone- or growth factor-like molecules, portions that span the cell membrane (transmembrane domain), and portions within the cell (intracellular
domain) that contain the enzymatic activity. In addition to kinase
activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are parts of a two-component signal transduction mechanisms. Described in greater detail below, essentially a HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on a 'receiver domain' on a different protein (or sometimes on the kinase itself) -- that aspartyl phosphate residue is thus activated for signaling.
In terms of enzymology, a histidine kinase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are ATP
and protein L-histidine
, whereas its two products
are ADP
and protein N-phospho-L-histidine.
Other names in common use include EnvZ, histidine kinase (ambiguous), HK1, HP165, and Sln1p. This enzyme participates in 4 metabolic pathways
: two-component system - general, bacterial chemotaxis - general, bacterial chemotaxis - organism-specific, and type ii secretion system.
ic unit may rotate in such a way that the ATP binding pocket of that unit can come into contact with a particular histidine residue on the opposite unit and a nucleophilic addition results in a phosphorylated histidine.
starting with a short N-terminal cytoplasmic portion connected to an extracellular sensing domain via a transmembrane α helix
. A second transmembrane α helix connects the extracellular domain to the C-terminal cytoplasmic catalytic domain. HKs are known to serve roles in many different signal transduction pathways, so it is not surprising that the extracellular sensing domain is not very well conserved in the HK family. In contrast, the cytoplasmic domain tends to have high sequence homology
and contains several well-known motifs
. These motifs include the H, N, G1, F, and G2 boxes. The autophosphorylation H-box is contained in the N-terminal dimerization and histidine phosphotransfer (DHp) domain. In HK853-CD, crystallized from Thermotoga maritima
, this domain is a helical-hairpin
and is formed by residues 232-317. The histidine phosphorylation site is located at His-260. The N, G1, F and G2 boxes are contained in the C-terminal catalytic and ATP-binding (CA) domain. This domain is formed by residues 323-489 and forms a structure known as an α/β sandwich fold. This particular fold has one layer composed of a 5-stranded β sheet
and the other layer is made of three α helices.
The dimeric unit is held together by a four-helix bundle, formed when the C-terminal segments of the α1 helices on each subunit interact in an antiparallel
manner with both α2 helices. The stability of the dimer is aided by several interactions at the interface between the DHps of each monomer. These include hydrophobic interactions between conserved hydrophobic
residues as well as two hydrogen bond
s (Thr-252...Glu-316’ and Arg-263...Asn-307’) and one salt bridge
(Lys-270...Glu-303’). Further interactions are mediated via hydrogen bonds to water within a cavity inside the coiled coil and flanked by hydrophobic residues.

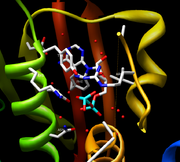 The nucleotide
The nucleotide
/ATP
binding pocket is contained within the CA domain and the structural similarity of this pocket is high between most HKs. The cavity of CheA, also crystallized from T. maritima, is first formed by β sheet P4 in the rear and the sides of the cavity are formed by the 4 motifs mentioned earlier, the N, G1, F, and G2 boxes. The majority of the residues coming from the β sheet are hydrophobic with Asp449 being the exception. This residue is invariant and forms a hydrogen bond along with a water molecule to the adenine
amine group. Three other water molecules form direct hydrogen bonds with the adenine base. A Mg2+ ion forms a bridge between all three phosphates and an invariant Asn residue. Finally, two more water molecules complete octahedral coordination with Mg2+ and are linked to Arg-408 and His-405. When the γ phosphate of ATP is destabilized, the Mg2+ is no longer observed due to its inability to octahedrally coordinate. Marina et al. argue that similar coordination of Mg2+ occurs in HK853 but that it is unobserved due to the usage of the ATP analog
AMPPNP in the crystal structure. During crystallization, the analog was hydrolyzed into a product similar to ADP.
The final side of the ATP binding pocket is conveniently named the “ATP lid.” The stability of this structure is mediated by the presence of the γ phosphate and thus the Mg2+ ion in the binding site. Also the presence of the nucleotide base has proved to play a significant role in stabilization of the lid in a closed conformation
. The ATP lid is connected via hydrophobic residues to the rest of the protein. The γ phosphate of ATP is somewhat exposed allowing for dephosphorylation
.
Upon ATP binding in this pocket, it is believed that a conformational change occurs allowing the rotation of the CA domain to come into contact with the DHp of the other monomer and thus allowing the conserved His-260 to rest near the γ phosphate. The Nε of His-260 then attacks the γ phosphate of ATP in a nucleophilic addition
and bumps off ADP
as its leaving group.
, which is often responsible for causing candidiasis
in immunocompromised
persons. C. albicans with a deletion of CHK1, the two-component histidine kinase gene, show defects in morphogenesis
and a drastic decrease in the cell’s ability to resist elimination by human neutrophils
. As humans lack this two-component system, it may be a good target for anti-microbial
agents in order to treat candidiasis
.
have been solved for this class of enzymes, with PDB
accession codes , , , , , , , , and .
Transferase
In biochemistry, a transferase is an enzyme that catalyzes the transfer of a functional group from one molecule to another . For example, an enzyme that catalyzed this reaction would be a transferase:In this example, A would be the donor, and B would be the acceptor...
class that play a role in signal transduction
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase
Autocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction....
, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell (extracellular
Extracellular
In cell biology, molecular biology and related fields, the word extracellular means "outside the cell". This space is usually taken to be outside the plasma membranes, and occupied by fluid...
domain) that bind to hormone- or growth factor-like molecules, portions that span the cell membrane (transmembrane domain), and portions within the cell (intracellular
Intracellular
Not to be confused with intercellular, meaning "between cells".In cell biology, molecular biology and related fields, the word intracellular means "inside the cell".It is used in contrast to extracellular...
domain) that contain the enzymatic activity. In addition to kinase
Kinase
In chemistry and biochemistry, a kinase is a type of enzyme that transfers phosphate groups from high-energy donor molecules, such as ATP, to specific substrates, a process referred to as phosphorylation. Kinases are part of the larger family of phosphotransferases...
activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are parts of a two-component signal transduction mechanisms. Described in greater detail below, essentially a HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on a 'receiver domain' on a different protein (or sometimes on the kinase itself) -- that aspartyl phosphate residue is thus activated for signaling.
In terms of enzymology, a histidine kinase is an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- ATP + protein L-histidine
 ADP + protein N-phospho-L-histidine.
ADP + protein N-phospho-L-histidine.
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
and protein L-histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
and protein N-phospho-L-histidine.
Other names in common use include EnvZ, histidine kinase (ambiguous), HK1, HP165, and Sln1p. This enzyme participates in 4 metabolic pathways
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
: two-component system - general, bacterial chemotaxis - general, bacterial chemotaxis - organism-specific, and type ii secretion system.
Mechanism
The mechanism for the reactions catalyzed by histidine kinase have not been completely elucidated, but current evidence suggests that the catalytic domain of one dimerProtein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
ic unit may rotate in such a way that the ATP binding pocket of that unit can come into contact with a particular histidine residue on the opposite unit and a nucleophilic addition results in a phosphorylated histidine.
Structure and function
An HK is composed of several domainsProtein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
starting with a short N-terminal cytoplasmic portion connected to an extracellular sensing domain via a transmembrane α helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
. A second transmembrane α helix connects the extracellular domain to the C-terminal cytoplasmic catalytic domain. HKs are known to serve roles in many different signal transduction pathways, so it is not surprising that the extracellular sensing domain is not very well conserved in the HK family. In contrast, the cytoplasmic domain tends to have high sequence homology
Homology (chemistry)
In chemistry, homology refers to the appearance of homologues. A homologue is a compound belonging to a series of compounds differing from each other by a repeating unit, such as a methylene group, a peptide residue, etcetera....
and contains several well-known motifs
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
. These motifs include the H, N, G1, F, and G2 boxes. The autophosphorylation H-box is contained in the N-terminal dimerization and histidine phosphotransfer (DHp) domain. In HK853-CD, crystallized from Thermotoga maritima
Thermotogae
Thermotogae is a phylum of the domain "Bacteria". This phylum comprises merely the class "Thermotogae", with the order "Thermotogales" and the family "Thermotogaceae"....
, this domain is a helical-hairpin
Stem-loop
Stem-loop intramolecular base pairing is a pattern that can occur in single-stranded DNA or, more commonly, in RNA. The structure is also known as a hairpin or hairpin loop. It occurs when two regions of the same strand, usually complementary in nucleotide sequence when read in opposite directions,...
and is formed by residues 232-317. The histidine phosphorylation site is located at His-260. The N, G1, F and G2 boxes are contained in the C-terminal catalytic and ATP-binding (CA) domain. This domain is formed by residues 323-489 and forms a structure known as an α/β sandwich fold. This particular fold has one layer composed of a 5-stranded β sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
and the other layer is made of three α helices.
The dimeric unit is held together by a four-helix bundle, formed when the C-terminal segments of the α1 helices on each subunit interact in an antiparallel
Antiparallel (biochemistry)
In biochemistry, two molecules are antiparallel if they run side-by-side in opposite directions or when both strands are complimentary to each other....
manner with both α2 helices. The stability of the dimer is aided by several interactions at the interface between the DHps of each monomer. These include hydrophobic interactions between conserved hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
residues as well as two hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s (Thr-252...Glu-316’ and Arg-263...Asn-307’) and one salt bridge
Salt bridge
A salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell...
(Lys-270...Glu-303’). Further interactions are mediated via hydrogen bonds to water within a cavity inside the coiled coil and flanked by hydrophobic residues.


Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
/ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
binding pocket is contained within the CA domain and the structural similarity of this pocket is high between most HKs. The cavity of CheA, also crystallized from T. maritima, is first formed by β sheet P4 in the rear and the sides of the cavity are formed by the 4 motifs mentioned earlier, the N, G1, F, and G2 boxes. The majority of the residues coming from the β sheet are hydrophobic with Asp449 being the exception. This residue is invariant and forms a hydrogen bond along with a water molecule to the adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
amine group. Three other water molecules form direct hydrogen bonds with the adenine base. A Mg2+ ion forms a bridge between all three phosphates and an invariant Asn residue. Finally, two more water molecules complete octahedral coordination with Mg2+ and are linked to Arg-408 and His-405. When the γ phosphate of ATP is destabilized, the Mg2+ is no longer observed due to its inability to octahedrally coordinate. Marina et al. argue that similar coordination of Mg2+ occurs in HK853 but that it is unobserved due to the usage of the ATP analog
Analog (chemistry)
In chemistry, a structural analog , also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced...
AMPPNP in the crystal structure. During crystallization, the analog was hydrolyzed into a product similar to ADP.
The final side of the ATP binding pocket is conveniently named the “ATP lid.” The stability of this structure is mediated by the presence of the γ phosphate and thus the Mg2+ ion in the binding site. Also the presence of the nucleotide base has proved to play a significant role in stabilization of the lid in a closed conformation
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
. The ATP lid is connected via hydrophobic residues to the rest of the protein. The γ phosphate of ATP is somewhat exposed allowing for dephosphorylation
Dephosphorylation
Dephosphorylation is the essential process of removing phosphate groups from an organic compound by hydrolysis. Its opposite is phosphorylation...
.
Upon ATP binding in this pocket, it is believed that a conformational change occurs allowing the rotation of the CA domain to come into contact with the DHp of the other monomer and thus allowing the conserved His-260 to rest near the γ phosphate. The Nε of His-260 then attacks the γ phosphate of ATP in a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
and bumps off ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
as its leaving group.
Role in fungal infections
A two-component system, involving histidine kinase and a variable response regulator protein, may be critical to the virulence of some fungal strains such as Candida albicansCandida albicans
Candida albicans is a diploid fungus that grows both as yeast and filamentous cells and a causal agent of opportunistic oral and genital infections in humans. Systemic fungal infections including those by C...
, which is often responsible for causing candidiasis
Candidiasis
Thrush redirects here. For the hoof infection see Thrush .Candidiasis or thrush is a fungal infection of any of the Candida species , of which Candida albicans is the most common...
in immunocompromised
Immunodeficiency
Immunodeficiency is a state in which the immune system's ability to fight infectious disease is compromised or entirely absent. Immunodeficiency may also decrease cancer immunosurveillance. Most cases of immunodeficiency are acquired but some people are born with defects in their immune system,...
persons. C. albicans with a deletion of CHK1, the two-component histidine kinase gene, show defects in morphogenesis
Morphogenesis
Morphogenesis , is the biological process that causes an organism to develop its shape...
and a drastic decrease in the cell’s ability to resist elimination by human neutrophils
Neutrophil granulocyte
Neutrophil granulocytes are the most abundant type of white blood cells in mammals and form an essential part of the innate immune system. They are generally referred to as either neutrophils or polymorphonuclear neutrophils , and are subdivided into segmented neutrophils and banded neutrophils...
. As humans lack this two-component system, it may be a good target for anti-microbial
Antimicrobial
An anti-microbial is a substance that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans. Antimicrobial drugs either kill microbes or prevent the growth of microbes...
agents in order to treat candidiasis
Candidiasis
Thrush redirects here. For the hoof infection see Thrush .Candidiasis or thrush is a fungal infection of any of the Candida species , of which Candida albicans is the most common...
.
Structural studies
As of late 2007, 9 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , , , , and .

