Heteropolymer
Encyclopedia
A heteropolymer or copolymer is a polymer
derived from two (or more) monomer
ic species, as opposed to a homopolymer where only one monomer is used. Copolymerization refers to methods used to chemically synthesize a copolymer.
Commercially relevant copolymers include ABS plastic, SBR, Nitrile rubber
, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate
.
 Since a copolymer consists of at least two types of constituent units (also structural unit
Since a copolymer consists of at least two types of constituent units (also structural unit
s), copolymers can be classified based on how these units are arranged along the chain. These include:
Copolymers may also be described in terms of the existence of or arrangement of branches in the polymer structure. Linear copolymers consist of a single main chain whereas branched copolymers consist of a single main chain with one or more polymeric side chains.
Other special types of branched copolymers include star copolymers, brush copolymers, and comb copolymers. In gradient copolymers
the monomer composition changes gradually along the chain.
A terpolymer is a copolymer consisting of three distinct monomer
s. The term is derived from ter (Latin), meaning thrice, and polymer
.
A special structure can be formed from one monomer where now the distinguishing feature is the tacticity
of each block.
For example, suppose we perform a free-radical polymerization
of styrene in the presence of polybutadiene
, a synthetic rubber
, which retains one reactive C=C double bond
per residue
. We get polystyrene
chains growing out in either direction from some of the places where there were double bonds, with a one-carbon rearrangement. Or to look at it the other way around, the result is a polystyrene backbone with polybutadiene chains growing out of it in both directions. This is an interesting copolymer variant in that one of the ingredients was a polymer to begin with.
As with block copolymers, the quasi-composite
product has properties of both "components". In the example cited, the rubbery chains absorb energy when the substance is hit, so it is much less brittle than ordinary polystyrene. The product is called high-impact polystyrene, or HIPS.
) and is usually made by first polymerizing styrene, and then subsequently polymerizing MMA from the reactive end of the polystyrene chains. This polymer is a "diblock copolymer" because it contains two different chemical blocks. Triblocks, tetrablocks, multiblocks, etc. can also be made. Diblock copolymers are made using living polymerization
techniques, such as atom transfer free radical polymerization (ATRP
), reversible addition fragmentation chain transfer (RAFT
), ring-opening metathesis polymerization (ROMP), and living cationic or living anionic polymerization
s. An emerging technique is chain shuttling polymerization
. The most powerful strategy to prepare block copolymers is the chemoselective stepwise coupling between polymeric precursors and heterofunctional linking agents. This method enables access to peculiarly exotic structures such as tetrablock quarterpolymers ABCD.
Recent research in block copolymers suggests that they may be useful in creating self-constructing fabrics with potential utility in semiconductor arrays (for example, computer memory devices) by assembling fine details atop a structured base created using conventional microlithography methods.
 Block copolymers are interesting because they can "microphase separate" to form periodic nanostructures, as in the styrene-butadiene-styrene block copolymer shown at right. The polymer is known as Kraton
Block copolymers are interesting because they can "microphase separate" to form periodic nanostructures, as in the styrene-butadiene-styrene block copolymer shown at right. The polymer is known as Kraton
and is used for shoe soles and adhesive
s. Owing to the microfine structure, the transmission electron microscope or TEM
was needed to examine the structure. The butadiene matrix was stained with osmium tetroxide to provide contrast in the image. The material was made by living polymerization
so that the blocks are almost monodisperse
, so helping to create a very regular microstructure. The molecular weight of the polystyrene blocks in the main picture is 102,000; the inset picture has a molecular weight of 91,000, producing slightly smaller domains.
 Microphase separation is a situation similar to that of oil
Microphase separation is a situation similar to that of oil
and water
. Oil and water are immiscible - they phase separate. Due to incompatibility between the blocks, block copolymers undergo a similar phase separation. Because the blocks are covalently bonded to each other, they cannot demix macroscopically as water and oil. In "microphase separation" the blocks form nanometer-sized structures. Depending on the relative lengths of each block, several morphologies can be obtained. In diblock copolymers, sufficiently different block lengths lead to nanometer-sized spheres of one block in a matrix of the second (for example PMMA in polystyrene). Using less different block lengths, a "hexagonally packed cylinder" geometry can be obtained. Blocks of similar length form layers (often called lamellae in the technical literature). Between the cylindrical and lamellar phase is the gyroid
phase. The nanoscale structures created from block copolymers could potentially be used for creating devices for use in computer memory
, nanoscale-templating and nanoscale separations.
Polymer scientists use thermodynamics
to describe how the different blocks interact. The product of the degree of polymerization, n, and the Flory-Huggins interaction parameter, , gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product
, gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product  is greater than 10.5. If
is greater than 10.5. If  is less than 10.5, the blocks will mix and microphase separation is not observed.
is less than 10.5, the blocks will mix and microphase separation is not observed.
also called the copolymerization equation:

where r1 = k11/k12 & r2 = k22/k21
. Elastomeric phases within a rigid matrix act as crack arrestors, and so increase the energy absorption when the material is impacted for example. Acrylonitrile butadiene styrene
is a common example.
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
derived from two (or more) monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
ic species, as opposed to a homopolymer where only one monomer is used. Copolymerization refers to methods used to chemically synthesize a copolymer.
Commercially relevant copolymers include ABS plastic, SBR, Nitrile rubber
Nitrile rubber
Nitrile rubber, also known as Buna-N, Perbunan, or NBR, is a synthetic rubber copolymer of acrylonitrile and butadiene. Trade names include Nipol, Krynac and Europrene....
, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate
Ethylene-vinyl acetate
Ethylene vinyl acetate is the copolymer of ethylene and vinyl acetate. The weight percent vinyl acetate usually varies from 10 to 40%, with the remainder being ethylene....
.
Types of copolymers

Structural unit
In polymer chemistry, a structural unit is a building block of a polymer chain. It is the result of a monomer which has been polymerized into a long chain....
s), copolymers can be classified based on how these units are arranged along the chain. These include:
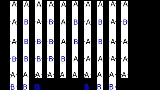
- Alternating copolymers with regular alternating A and B units (2)
- Periodic copolymers with A and B units arranged in a repeating sequence (e.g. (A-B-A-B-B-A-A-A-A-B-B-B)n)
- Statistical copolymers are copolymers in which the sequence of monomer residues follows a statistical rule. If the probability of finding a given type monomer residue at a particular point in the chain is equal to the mole fraction of that monomer residue in the chain, then the polymer may be referred to as a truly random copolymer (3).
- Block copolymers comprise two or more homopolymer subunits linked by covalent bonds (4). The union of the homopolymer subunits may require an intermediate non-repeating subunit, known as a junction block. Block copolymers with two or three distinct blocks are called diblock copolymers and triblock copolymers, respectively.
Copolymers may also be described in terms of the existence of or arrangement of branches in the polymer structure. Linear copolymers consist of a single main chain whereas branched copolymers consist of a single main chain with one or more polymeric side chains.
Other special types of branched copolymers include star copolymers, brush copolymers, and comb copolymers. In gradient copolymers
Gradient copolymers
Copolymers are polymers that are synthesized with more than one kind of repeat unit . It exhibits a gradual change in monomer composition from predominantly one species to predominantly the other, unlike with block copolymers, which have an abrupt change in composition, and random copolymers,...
the monomer composition changes gradually along the chain.
A terpolymer is a copolymer consisting of three distinct monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s. The term is derived from ter (Latin), meaning thrice, and polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
.
- Stereoblock copolymers
A special structure can be formed from one monomer where now the distinguishing feature is the tacticity
Tacticity
Tacticity is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects of tacticity on the physical properties of the polymer...
of each block.
Graft copolymers
Graft copolymers are a special type of branched copolymer in which the side chains are structurally distinct from the main chain. The illustration (5) depicts a special case where the main chain and side chains are composed of distinct homopolymers. However, the individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.For example, suppose we perform a free-radical polymerization
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
of styrene in the presence of polybutadiene
Polybutadiene
Polybutadiene is a synthetic rubber that is a polymer formed from the polymerization process of the monomer 1,3-butadiene.It has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production...
, a synthetic rubber
Synthetic rubber
Synthetic rubber is is any type of artificial elastomer, invariably a polymer. An elastomer is a material with the mechanical property that it can undergo much more elastic deformation under stress than most materials and still return to its previous size without permanent deformation...
, which retains one reactive C=C double bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
per residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
. We get polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
chains growing out in either direction from some of the places where there were double bonds, with a one-carbon rearrangement. Or to look at it the other way around, the result is a polystyrene backbone with polybutadiene chains growing out of it in both directions. This is an interesting copolymer variant in that one of the ingredients was a polymer to begin with.
As with block copolymers, the quasi-composite
Composite material
Composite materials, often shortened to composites or called composition materials, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct at the macroscopic or...
product has properties of both "components". In the example cited, the rubbery chains absorb energy when the substance is hit, so it is much less brittle than ordinary polystyrene. The product is called high-impact polystyrene, or HIPS.
Block copolymers
A special kind of copolymer is called a "block copolymer". Block copolymers are made up of blocks of different polymerized monomers. For example, PS-b-PMMA is short for polystyrene-b-poly(methyl methacrylateMethyl methacrylate
Methyl methacrylate is an organic compound with the formula CH2=CCOOCH3. This colourless liquid, the methyl ester of methacrylic acid is a monomer produced on a large scale for the production of poly .-Production:...
) and is usually made by first polymerizing styrene, and then subsequently polymerizing MMA from the reactive end of the polystyrene chains. This polymer is a "diblock copolymer" because it contains two different chemical blocks. Triblocks, tetrablocks, multiblocks, etc. can also be made. Diblock copolymers are made using living polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
techniques, such as atom transfer free radical polymerization (ATRP
ATRP (chemistry)
Atom transfer radical polymerization is an example of a living polymerization or a controlled/living radical polymerization . Like its counter part, ATRA or atom transfer radical addition, it is a means of forming carbon-carbon bond through transition metal catalyst...
), reversible addition fragmentation chain transfer (RAFT
RAFT (chemistry)
Reversible Addition-Fragmentation chain Transfer or RAFT polymerization is one kind of controlled radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation in 1998, RAFT polymerization is a relatively new method for the synthesis of living radical...
), ring-opening metathesis polymerization (ROMP), and living cationic or living anionic polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
s. An emerging technique is chain shuttling polymerization
Chain shuttling polymerization
Chain Shuttling Polymerization is a dual-catalyst method for producing block copolymers with alternating or variable tacticity. The desired effect of this method is to generate hybrid polymers that bear the properties of both polymer chains, such as a high melting point accompanied by high...
. The most powerful strategy to prepare block copolymers is the chemoselective stepwise coupling between polymeric precursors and heterofunctional linking agents. This method enables access to peculiarly exotic structures such as tetrablock quarterpolymers ABCD.
Recent research in block copolymers suggests that they may be useful in creating self-constructing fabrics with potential utility in semiconductor arrays (for example, computer memory devices) by assembling fine details atop a structured base created using conventional microlithography methods.
Phase separation

Kraton (polymer)
Kraton is the trade name given to a number of high performance elastomers manufactured by Kraton Polymers , and used as synthetic replacements for rubber. Kraton polymers offers many of the properties of natural rubber, such as flexibility, high traction, and sealing abilities, but with increased...
and is used for shoe soles and adhesive
Adhesive
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials...
s. Owing to the microfine structure, the transmission electron microscope or TEM
Transmission electron microscopy
Transmission electron microscopy is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through...
was needed to examine the structure. The butadiene matrix was stained with osmium tetroxide to provide contrast in the image. The material was made by living polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
so that the blocks are almost monodisperse
Monodisperse
A collection of objects are called monodisperse, or monosized, if they have the same size and shape when discussing particles, and the same mass when discussing polymers...
, so helping to create a very regular microstructure. The molecular weight of the polystyrene blocks in the main picture is 102,000; the inset picture has a molecular weight of 91,000, producing slightly smaller domains.

Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. Oil and water are immiscible - they phase separate. Due to incompatibility between the blocks, block copolymers undergo a similar phase separation. Because the blocks are covalently bonded to each other, they cannot demix macroscopically as water and oil. In "microphase separation" the blocks form nanometer-sized structures. Depending on the relative lengths of each block, several morphologies can be obtained. In diblock copolymers, sufficiently different block lengths lead to nanometer-sized spheres of one block in a matrix of the second (for example PMMA in polystyrene). Using less different block lengths, a "hexagonally packed cylinder" geometry can be obtained. Blocks of similar length form layers (often called lamellae in the technical literature). Between the cylindrical and lamellar phase is the gyroid
Gyroid
"Gyroid" redirects here. For the creature, see Animal Crossing .A gyroid is a certain infinitely connected triply periodic minimal surface discovered by Alan Schoen in 1970.The gyroid has space group Iad...
phase. The nanoscale structures created from block copolymers could potentially be used for creating devices for use in computer memory
Memory
In psychology, memory is an organism's ability to store, retain, and recall information and experiences. Traditional studies of memory began in the fields of philosophy, including techniques of artificially enhancing memory....
, nanoscale-templating and nanoscale separations.
Polymer scientists use thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
to describe how the different blocks interact. The product of the degree of polymerization, n, and the Flory-Huggins interaction parameter,
 , gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product
, gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product  is greater than 10.5. If
is greater than 10.5. If  is less than 10.5, the blocks will mix and microphase separation is not observed.
is less than 10.5, the blocks will mix and microphase separation is not observed.Copolymer equation
An alternating copolymer has the formula: -A-B-A-B-A-B-A-B-A-B-, or -(-A-B-)n-. The molar ratios of the monomer in the polymer is close to one, which happens when the reactivity ratios r1 & r2 are close to zero, as given by the Mayo-Lewis equationMayo-Lewis equation
The Mayo-Lewis equation or copolymer equation in polymer chemistry describes the distribution of monomers in a copolymer :Taking into consideration a monomer mix of two components M_1\, and M_2\, and the four different reactions that can take place at the reactive chain end terminating in either...
also called the copolymerization equation:

where r1 = k11/k12 & r2 = k22/k21
Copolymer engineering
Copolymerization is used to modify the properties of man-made plastics to specific needs, for example to reduce crystallinity, modify glass transition temperature or to improve solubility. It is a way of improving mechanical properties, in a technique known as rubber tougheningRubber toughening
Many thermoplastics such as polystyrene and PMMA are brittle when stressed, a property which limits applications. A good way of strengthening such polymers is to copolymerise elastomeric chains during manufacture...
. Elastomeric phases within a rigid matrix act as crack arrestors, and so increase the energy absorption when the material is impacted for example. Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene is a common thermoplastic. Its melting point is approximately 105 °C ....
is a common example.
See also
- Copolymers section of Polymer article
- Thermoplastic elastomerThermoplastic elastomerThermoplastic elastomers , sometimes referred to as thermoplastic rubbers, are a class of copolymers or a physical mix of polymers which consist of materials with both thermoplastic and elastomeric properties...
- TholinTholinTholin [after the ancient Greek word meaning "not clear"] is a heteropolymer molecule formed by solar ultraviolet irradiation of simple organic compounds such as methane or ethane. Tholins do not form naturally on modern-day Earth, but are found in great abundance on the surface of icy bodies in...

