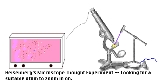
Heisenberg's microscope
Encyclopedia
Heisenberg's microscope exists only as a thought experiment
, one that was proposed by Werner Heisenberg
, criticized by his mentor Niels Bohr
, and subsequently served as the nucleus of some commonly held ideas, and misunderstandings, about Quantum Mechanics. In particular, it provided an argument for the uncertainty principle
on the basis of the principles of classical optics
.
 direction along a line below the microscope, as in the illustration to the right. Let the cone of light rays leaving the microscope lens and focusing on the electron makes an angle
direction along a line below the microscope, as in the illustration to the right. Let the cone of light rays leaving the microscope lens and focusing on the electron makes an angle  with the electron. Let
with the electron. Let 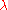 be the wavelength of the light rays. Then, according to the laws of classical optics, the microscope can only resolve
be the wavelength of the light rays. Then, according to the laws of classical optics, the microscope can only resolve
the position of the electron up to an accuracy of

When an observer perceives an image of the particle, it's because the light rays strike the particle and bounce back through the microscope to their eye. However, we know from experience that when a photon strikes an electron, the latter has a Compton recoil
with momentum proportional to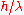 , where
, where 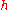 is Planck's constant
is Planck's constant
. It is at this point that Heisenberg introduces objective indeterminacy into the thought experiment. He writes that "the recoil cannot be exactly known, since the direction of the scattered photon is undetermined within the bundle of rays entering the microscope" (p.21). In particular, the electron's momentum in the direction is only determined up to
direction is only determined up to

Combining the relations for and
and  , we thus have that
, we thus have that
 ,
,
which is an approximate expression of Heisenberg's uncertainty principle
.
, which stands as one of the pillars of modern physics and as a theory that has been tested and confirmed countless times. That being said, the thought experiment has the somewhat unusual characteristic of attacking the premises under which it was constructed (Reductio ad absurdum
), or at least of being involved in the development of an area of physics, quantum mechanics, that redefined the terms under which the original thought experiment was conceived. Quantum mechanics questions whether electrons actually have a determinate position before they are disturbed by the measurement that one might try to use to establish that they have such determinate positions. Under a more thorough quantum mechanical analysis, an electron has some probability of showing up at any point in the universe, but the probability that it will be far from where one might expect it to be becomes very low for places at great distances from the neighborhood in which it was originally found. In other words, the "position" of an electron can only be stated in terms of a probability distribution, and predictions of where it will move to can also only be given in terms of a probability distribution.
Thought experiment
A thought experiment or Gedankenexperiment considers some hypothesis, theory, or principle for the purpose of thinking through its consequences...
, one that was proposed by Werner Heisenberg
Werner Heisenberg
Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory...
, criticized by his mentor Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
, and subsequently served as the nucleus of some commonly held ideas, and misunderstandings, about Quantum Mechanics. In particular, it provided an argument for the uncertainty principle
Uncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
on the basis of the principles of classical optics
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
.
Heisenberg's argument
Heisenberg's argument can be found in (Heisenberg 1930), and is summarized as follows. Heisenberg begins by supposing that an electron is like a classical particle, moving in the direction along a line below the microscope, as in the illustration to the right. Let the cone of light rays leaving the microscope lens and focusing on the electron makes an angle
direction along a line below the microscope, as in the illustration to the right. Let the cone of light rays leaving the microscope lens and focusing on the electron makes an angle  with the electron. Let
with the electron. Let 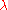 be the wavelength of the light rays. Then, according to the laws of classical optics, the microscope can only resolve
be the wavelength of the light rays. Then, according to the laws of classical optics, the microscope can only resolveOptical resolution
Optical resolution describes the ability of an imaging system to resolve detail in the object that is being imaged.An imaging system may have many individual components including a lens and recording and display components...
the position of the electron up to an accuracy of

When an observer perceives an image of the particle, it's because the light rays strike the particle and bounce back through the microscope to their eye. However, we know from experience that when a photon strikes an electron, the latter has a Compton recoil
Compton wavelength
The Compton wavelength is a quantum mechanical property of a particle. It was introduced by Arthur Compton in his explanation of the scattering of photons by electrons...
with momentum proportional to
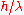 , where
, where 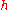 is Planck's constant
is Planck's constantPlanck constant
The Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
. It is at this point that Heisenberg introduces objective indeterminacy into the thought experiment. He writes that "the recoil cannot be exactly known, since the direction of the scattered photon is undetermined within the bundle of rays entering the microscope" (p.21). In particular, the electron's momentum in the
 direction is only determined up to
direction is only determined up to
Combining the relations for
 and
and  , we thus have that
, we thus have that ,
,which is an approximate expression of Heisenberg's uncertainty principle
Uncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
.
Problems with the argument
This thought experiment was formulated to help in introducing Heisenberg's Uncertainty PrincipleUncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
, which stands as one of the pillars of modern physics and as a theory that has been tested and confirmed countless times. That being said, the thought experiment has the somewhat unusual characteristic of attacking the premises under which it was constructed (Reductio ad absurdum
Reductio ad absurdum
In logic, proof by contradiction is a form of proof that establishes the truth or validity of a proposition by showing that the proposition's being false would imply a contradiction...
), or at least of being involved in the development of an area of physics, quantum mechanics, that redefined the terms under which the original thought experiment was conceived. Quantum mechanics questions whether electrons actually have a determinate position before they are disturbed by the measurement that one might try to use to establish that they have such determinate positions. Under a more thorough quantum mechanical analysis, an electron has some probability of showing up at any point in the universe, but the probability that it will be far from where one might expect it to be becomes very low for places at great distances from the neighborhood in which it was originally found. In other words, the "position" of an electron can only be stated in terms of a probability distribution, and predictions of where it will move to can also only be given in terms of a probability distribution.
Footnotes
¹ Heisenberg, excerpt giving his own description of this thought experiment in The World of Mathematics, II, p. 1052.Sources
- Amir D. Aezel, Entanglement, pp. 77-79.
- Niels Bohr, Nature, 121, p. 580, 1928.
- Werner Heisenberg, Physics and Philosophy, pp. 46ff.
- Werner Heisenberg, The Physical Principles of the Quantum Theory, 1930.
- Albert Messiah, Quantum Mechanics, I, p. 143f
- James R. Newman, ed., The World of Mathematics, II, pp. 1051-1055
External links
See also
- Quantum mechanicsQuantum mechanicsQuantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
- Basics of quantum mechanics
- Interpretation of quantum mechanicsInterpretation of quantum mechanicsAn interpretation of quantum mechanics is a set of statements which attempt to explain how quantum mechanics informs our understanding of nature. Although quantum mechanics has held up to rigorous and thorough experimental testing, many of these experiments are open to different interpretations...
- Philosophical interpretation of classical physicsPhilosophical interpretation of classical physicsClassical Newtonian physics has, formally, been replaced by quantum mechanics on the small scale and relativity on the large scale. Because most humans continue to think in terms of the kind of events we perceive in the human scale of daily life, it became necessary to provide a new philosophical...
- Schrödinger's catSchrödinger's catSchrödinger's cat is a thought experiment, usually described as a paradox, devised by Austrian physicist Erwin Schrödinger in 1935. It illustrates what he saw as the problem of the Copenhagen interpretation of quantum mechanics applied to everyday objects. The scenario presents a cat that might be...
- Uncertainty principleUncertainty principleIn quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
- Quantum field theoryQuantum field theoryQuantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and...
- Electromagnetic radiationElectromagnetic radiationElectromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...

