
Ground glass joint
Encyclopedia


Oil bubbler
An gas bubbler is a piece of laboratory glassware which consists of a glass bulb filled with a small amount of fluid — usually mineral or silicone oil, less commonly mercury. The inlet to the bulb is connected to a ground glass joint, while the outlet is vented to the air.Gas bubblers are...
with ground glass joints may be rapidly fitted together to reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
a reaction mixture. This is a large improvement compared with older methods of custom-made glassware, which was time-consuming and expensive, or the use of less chemically- and heat-resistant cork
Cork (material)
Cork is an impermeable, buoyant material, a prime-subset of bark tissue that is harvested for commercial use primarily from Quercus suber , which is endemic to southwest Europe and northwest Africa...
s or rubber bungs and glass tubes as joints which took time to prepare as well.
To connect the hollow inner spaces of the glassware components, ground glass joints are hollow on the inside and open at the ends, except for stoppers.
History
Crude versions of conically-tapered ground glass joints have been made for quite a while, particularly for stoppers for glass bottleBottle
A bottle is a rigid container with a neck that is narrower than the body and a "mouth". By contrast, a jar has a relatively large mouth or opening. Bottles are often made of glass, clay, plastic, aluminum or other impervious materials, and typically used to store liquids such as water, milk, soft...
s and retort
Retort
In a chemistry laboratory, a retort is a glassware device used for distillation or dry distillation of substances. It consists of a spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heated...
s. These days, ground glass joints can be precisely ground to a reproducible taper or shape. They are made to join two glassware pieces together. One of the glassware items to be joined would have an inner (or male) joint with the ground glass surface facing outward and the other would have an outer (or female) joint of a correspondingly-fitting taper with the ground glass
Ground glass
Ground glass is glass whose surface has been ground to produce a flat but rough finish.Ground glass surfaces have many applications, ranging from mere ornamentation on windows and table glassware to scientific uses in optics and laboratory glassware....
surface facing inward.
Joint types
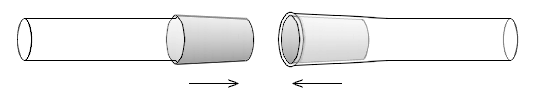
Two general types of ground glass joints are fairly commonly used: joints which are slightly conically-tapered and ball and socket joints (sometimes called spherical joints).Conically tapered joints

United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
. There are also Europe
Europe
Europe is, by convention, one of the world's seven continents. Comprising the westernmost peninsula of Eurasia, Europe is generally 'divided' from Asia to its east by the watershed divides of the Ural and Caucasus Mountains, the Ural River, the Caspian and Black Seas, and the waterways connecting...
an ISO standard joints with common joint sizes of 10/19, 14/23, 19/26, 24/29 and 29/32. The US and ISO joints differ only in the length not in the slope, and some can be used in combination. The stopper joints of chemical bottles, volumetric flask
Volumetric flask
A volumetric flask is a piece of laboratory glassware, a type of laboratory flask, used in analytical chemistry for the preparation of solutions. It is made of glass or plastic and consists of a flat bottomed bulb with a long neck, usually fitted with a stopper. The stopper is normally made in a...
s, and separatory funnel
Separatory funnel
A separatory funnel, also known as separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate the components of a mixture between two immiscible solvent phases of different densities Typically, one of the...
s often do not use the precision standard taper joints. Stopper joints are designated (if at all) only by the maximum diameter number.
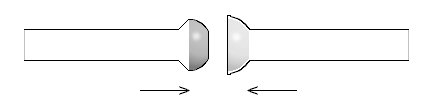
Ball-and-socket joints
For ball-and-socket joints (also known as spherical joints), the inner joint is a ball and the outer joint is a socket, both having holes leading to the interior of their respective tube ends, to which they are fused. The ball tip is a hemisphere with a ground-glass surface on the outside, which fits inside of the socket, where the ground glass surface is on the inside. Ball-and-socket joints are labeled with a size code consisting of a number, a slash, and another number. The first number represents the outer diameter in mm of the ball at its base or the inner diameter in millimeters at the tip of the socket, in both cases where the diameters are their maximum in the joints.The second number represents the inner diameter of the hole in the middle of the ball or socket, which leads to the inner diameter of the tube fused to the joint.
They can also be found as the necks on pilot plant production flasks, where large volumes and masses are present, and on some Schlenk line
Schlenk line
225px|thumb|Vacuum gas manifold set up: 1 inert gas in, 2 inert gas out , 3 vacuum 4 reaction line, 5 Teflon tap to gas, 6 Teflon tap to vacuum 225px|thumb| Vacuum gas manifold set up: 1 inert gas in, 2 inert gas out , 3 vacuum , 4 reaction line, 5 double oblique stopcock...
s, where the long spans of fine glass benefit from a little flexibility between pieces. Generally, when considering smaller glassware, ball and sockets are far outnumbered by standard tapers.
Adapters
For either standard taper joints or ball-and-socket joints, inner and outer joints with the same numbers are made to fit together. When the joint sizes are different, ground glass adapters may be available (or made) to place in between to connect them. Special clips or pinch clamps may be placed around the joints to hold them in place.Round-bottom flask
Round-bottom flask
Round-bottom flasks are types of flasks having spherical bottoms used as laboratory glassware, mostly for chemical or biochemical work. They are typically made of glass for chemical inertness; and in modern days, they are usually made of heat-resistant borosilicate glass...
s often have one or more conically-tapered ground glass joint openings or necks. Conventionally, these joints at the flask necks are outer joints. Other adapters, such as distillation heads and vacuum adapters, are made with joints that fit in with this convention. If a flask or other container has an extra outer ground-glass joint on it which needs to be closed off for an experiment, there are often conically-tapered inner ground-glass stoppers for that purpose. In some cases, small hook-like protrusions made of glass may be fused onto the rest of the glass item near a joint to allow an end loop of a small spring to be attached so the spring helps keep joints temporarily together. The use of a special very small size of conically-tapered fitting for glass, plastic, or metal parts called a Luer fitting
Luer Taper
The Luer taper is a standardized system of small-scale fluid fittings used for making leak-free connections between a male-taper fitting and its mating female part on medical and laboratory instruments, including hypodermic syringe tips and needles or stopcocks and needles...
or adapter has become more widespread. Originally, Luer fittings were used to connect the hub of a needle to a syringe
Syringe
A syringe is a simple pump consisting of a plunger that fits tightly in a tube. The plunger can be pulled and pushed along inside a cylindrical tube , allowing the syringe to take in and expel a liquid or gas through an orifice at the open end of the tube...
. Where the use of ground glass presents a problem, as in the production or distillation of diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
(which may explode on contact with rougher surfaces), equipment with smooth glass joints may be used.
Keck clips

Patented in 1984 by Hermann Keck, Keck clips are used to hold glass joints together. These are usually made of polyacetal, and are colored according to joint sizes.
Polyacetal melts at a reasonably low temperature (around 175°C) and begins to soften around 140°C. As glassware temperatures are recommended up to 250°C, care needs to be taken that clips made from this material are not being used to hold glass together that will get this hot. Typical problem areas include a flask over the plate (which may drop off the end of the column as it gets hot) and the connection the condenser makes to the still head (which will reach high temperatures and may allow the condenser to fall off). As such, different clips should be used at these points or the glassware should be clamped such that these elements can't slide apart or don't need the clip. Polyacetal clips suffer another problem in that the material is strongly affected by the corrosive gases. This effect can be so dramatic that the clip will fall apart in minutes of exposure to minute quantities leaking through even greased, ground tapers. Importantly, this failure mode is sudden and without warning.
PTFE is sometimes used, as its recommended temperature peak matches that of most practical chemistry work. Its highly inert nature also makes it immune to degradation around the corrosive gases. However, it is both expensive and will begin producing hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
if heated to beyond its specified temperature; so care must be taken to avoid this, given the level of risk the result presents. The same is true of using Krytox and chemically resistant Molykote (PTFE thickened, fluoro based) oils and greases for glassware seals.
Stainless steel is a final option. Naturally, this can withstand the entire temperature spectrum of borosilicate glass and is reasonably inert. Though, lower grades of stainless are still rapidly attacked in the presence of the corrosive gases and the clips themselves are often as expensive as PTFE.
Some glassware features barbs (Devil's horns / Viking helmet) sticking out the sides of the tapers. Small stainless steel springs are used on these to hold the joint together. The use of springs is of particular benefit when dealing with positive pressures, as they apply enough force for the glass to operate, but will open the taper if an unexpected excursion occurs. This method is considered quite old fashioned, but is still used on some of the most well known and high end glassware available.
Extreme consideration must be taken when using clips on glassware that will generate a positive pressure, as it can be very easy for passages to block when using reactive gases and dip tubes. Hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
, for example, has a vapour pressure at room temperature that is high enough to burst standard glassware. It is almost ubiquitously the case that only those joints that must be clipped should be clipped. It is safer to have an unclipped joint open and vent some of the corrosive gas than to have the same occur with the addition of the glassware exploding, emptying all of the contents and all of the gas out.
Lubrication and sealing

A rather thin layer of grease
Grease (lubricant)
The term grease is used to describe semisolid lubricants. Although the word grease is also used to describe rendered fat of animals, in the context of lubrication, grease typically applies to a material consisting of a soap emulsified with mineral or vegetable oil...
particularly made for this application can be applied to the ground glass surfaces to be connected and the inner joint is inserted into the outer joint such that the ground glass surfaces of each are next to each other to make the connection. In addition to making a leak-tight connection, the grease allows to joints to be later separated more easily. However, a potential drawback of using such grease is that if it is used on laboratory glassware
Laboratory glassware
Laboratory glassware refers to a variety of equipment, traditionally made of glass, used for scientific experiments and other work in science, especially in chemistry and biology laboratories...
for an extended period of time in high temperature applications (such as for continuous distillation
Continuous distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation or partial separation of a liquid feed mixture into components or...
), the grease may eventually cause contamination of the chemicals.
PTFE (Teflon) sleeves and PTFE sealing rings are used in between joints to fit them together instead of grease. PTFE tape is another alternative that is finding widespread use. Both PTFE tape, bands, chemically resistant Molykote & Krytox grease or oils all emit hydrogen fluoride fumes as they approach and exceed their working temperature limits, which can occur when using a hotplate, mantle, oil bath or flame.
Cleaning
Ground glass joints are translucent and physically free of debris when clean. Solvents, reaction mixtures, and old grease show up as transparent spots. Grease can be removed by wiping with an appropriate solvent; ether, methylene chloride, ethyl acetate, or hexanes work well for silicone- and hydrocarbon-based greases. Fluoroether-based greases are quite impervious to organic solvents; most chemists simply wipe them off as much as possible. That said, there are fluorinated solvents available which can remove fluoroether greases, but they are much more expensive than laboratory solvents.Frozen joints
Standard taper ground glass joints sometimes freeze or seize. Ball and socket-type joints are not susceptible, due to the larger freedom of motion of the joint. Joints may freeze for a few reasons, such as:- the lack of lubrication (with grease, sleeves, or tape)
- attack of the ground glass surfaces by strong bases (e.g., hydroxide, phosphate, or carbonate, dissolving SiO2 (i.e., silicic acid: H4SiO4 / Si(OH)4) from glass at high pH)
- deposition of solids from reaction mixtures
- leaching of greases by organic solvents
- dirt or other debris
- allowing sealed vessels to cool (creating a pressure difference across the joint)
Frozen joints may be removed by working solvent into the joint while rocking the stopper, heating the joint, or cooling the stopper. There are also specialized glassblower tools to deal with this problem.

