Grain growth
Encyclopedia
Grain growth is the increase in size of grains (crystallite
s) in a material at high temperature. This occurs when recovery
and recrystallisation
are complete and further reduction in the internal energy can only be achieved by reducing the total area of grain boundary. The term is commonly used in metallurgy but is also used in reference to ceramics and minerals.
. Since boundaries are regions of high energy they make excellent sites for the nucleation of precipitates and other second-phases e.g. Mg–Si–Cu phases in some aluminium alloys or martensite
platlets in steel. Depending on the second phase in question this may have positive or negative effects.
. Although such methods enabled the collection of a great deal of empirical evidence, particular with regard to factors such as temperature
or composition
, the lack of crystallographic information limited the development of an understanding of the fundamental physics
. Nevertheless, the following became well-established features of grain growth:
In comparison to phase transformations the energy available to drive grain growth is very low and so
it tends to occur at much slower rates and is easily slowed by particles or solute atoms.
the reduction of the total amount of grain boundary surface energy. Additional contributions to the driving force by e.g. elastic strains or temperature gradients are neglected. If it holds that the rate of growth is proportional to the driving force and that the driving force is proportional to the total amount of grain boundary energy, then it can be shown that the time t required to reach a given grain size is approximated by the equation
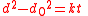
where d0 is the initial grain size, d is the final grain size and k is a temperature dependent constant given by an exponential law:

where k0 is a constant, T is the absolute temperature and Q is the activation energy for boundary mobility. Theoretically, the activation energy for boundary mobility should equal that for self-diffusion but this is often found not to be the case.
In general these equations are found to hold for ultra-high purity materials but rapidly fail when even tiny concentrations of solute are introduced.
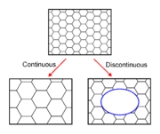
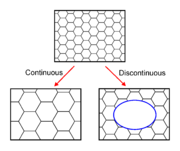 In common with recovery
In common with recovery
and recrystallisation
, growth phenomena can be separated into continuous and discontinuous mechanisms. In the former the microstructure evolves from state A to B (in this case the grains get larger) in a uniform manner. In the latter, the changes occur heterogeneously and specific transformed and untransformed regions may be identified. Discontinuous grain growth is characterised by a subset of grains growing at a high rate and at the expense of their neighbours and tends to result in a microstructure dominated by a few very large grains. In order for this to occur the subset of grains must possess some advantage over their competitors such as a high grain boundary energy, locally high grain boundary mobility, favourable texture or lower local second-phase particle density.
by particles, then the grain size may be restricted to a much lower value than might otherwise be expected. This is an important industrial mechanism in preventing the softening of materials at high temperature.
Crystallite
Crystallites are small, often microscopic crystals that, held together through highly defective boundaries, constitute a polycrystalline solid. Metallurgists often refer to crystallites as grains.- Details :...
s) in a material at high temperature. This occurs when recovery
Recovery (metallurgy)
Recovery is a process by which deformed grains can reduce their stored energy by the removal or rearrangement of defects in their crystal structure. These defects, primarily dislocations, are introduced by plastic deformation of the material and act to increase the yield strength of a material...
and recrystallisation
Recrystallization (metallurgy)
Recrystallization is a process by which deformed grains are replaced by a new set of undeformed grains that nucleate and grow until the original grains have been entirely consumed. Recrystallization is usually accompanied by a reduction in the strength and hardness of a material and a simultaneous...
are complete and further reduction in the internal energy can only be achieved by reducing the total area of grain boundary. The term is commonly used in metallurgy but is also used in reference to ceramics and minerals.
Importance of grain growth
Most materials exhibit the Hall–Petch effect at room-temperature and so display a higher yield stress when the grain size is reduced. At high temperatures the opposite is true since the open, disordered nature of grain boundaries means that vacancies can diffuse more rapidly down boundaries leading to more rapid Coble creepCoble creep
Coble creep, a form of diffusion creep, is a mechanism for deformation of crystalline solids. Coble creep occurs through the diffusion of atoms in a material along the grain boundaries, which produces a net flow of material and a sliding of the grain boundaries.Coble creep is named after Robert L...
. Since boundaries are regions of high energy they make excellent sites for the nucleation of precipitates and other second-phases e.g. Mg–Si–Cu phases in some aluminium alloys or martensite
Martensite
Martensite, named after the German metallurgist Adolf Martens , most commonly refers to a very hard form of steel crystalline structure, but it can also refer to any crystal structure that is formed by displacive transformation. It includes a class of hard minerals occurring as lath- or...
platlets in steel. Depending on the second phase in question this may have positive or negative effects.
Rules of grain growth
Grain growth has long been studied primarily by the examination of sectioned, polished and etched samples under the optical microscopeMicroscope
A microscope is an instrument used to see objects that are too small for the naked eye. The science of investigating small objects using such an instrument is called microscopy...
. Although such methods enabled the collection of a great deal of empirical evidence, particular with regard to factors such as temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
or composition
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
, the lack of crystallographic information limited the development of an understanding of the fundamental physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
. Nevertheless, the following became well-established features of grain growth:
- Grain growth occurs by the movement of grain boundaries and not by coalescence (i.e. like water droplets)
- Boundary movement is discontinuous and the direction of motion may change suddenly.
- One grain may grow into another grain whilst being consumed from the other side
- The rate of consumption often increases when the grain is nearly consumed
- A curved boundary typically migrates towards its centre of curvature
- When grain boundaries in a single phase meet at angles other than 120 degrees, the grain included by the more acute angle will be consumed so that the angles approach 120 degrees.
Driving force
The boundary between one grain and its neighbour is a defect in the crystal structure and so it is associated with a certain amount of energy. As a result there is a thermodynamic driving force for the total area of boundary to be reduced. If the grain size increases, accompanied by a reduction in the actual number of grains, then the total area of boundary will be reduced.In comparison to phase transformations the energy available to drive grain growth is very low and so
it tends to occur at much slower rates and is easily slowed by particles or solute atoms.
Ideal grain growth
Ideal grain growth is a special case of normal grain growth where boundary motion is driven only bythe reduction of the total amount of grain boundary surface energy. Additional contributions to the driving force by e.g. elastic strains or temperature gradients are neglected. If it holds that the rate of growth is proportional to the driving force and that the driving force is proportional to the total amount of grain boundary energy, then it can be shown that the time t required to reach a given grain size is approximated by the equation
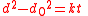
where d0 is the initial grain size, d is the final grain size and k is a temperature dependent constant given by an exponential law:

where k0 is a constant, T is the absolute temperature and Q is the activation energy for boundary mobility. Theoretically, the activation energy for boundary mobility should equal that for self-diffusion but this is often found not to be the case.
In general these equations are found to hold for ultra-high purity materials but rapidly fail when even tiny concentrations of solute are introduced.
Normal vs abnormal

Recovery (metallurgy)
Recovery is a process by which deformed grains can reduce their stored energy by the removal or rearrangement of defects in their crystal structure. These defects, primarily dislocations, are introduced by plastic deformation of the material and act to increase the yield strength of a material...
and recrystallisation
Recrystallization (metallurgy)
Recrystallization is a process by which deformed grains are replaced by a new set of undeformed grains that nucleate and grow until the original grains have been entirely consumed. Recrystallization is usually accompanied by a reduction in the strength and hardness of a material and a simultaneous...
, growth phenomena can be separated into continuous and discontinuous mechanisms. In the former the microstructure evolves from state A to B (in this case the grains get larger) in a uniform manner. In the latter, the changes occur heterogeneously and specific transformed and untransformed regions may be identified. Discontinuous grain growth is characterised by a subset of grains growing at a high rate and at the expense of their neighbours and tends to result in a microstructure dominated by a few very large grains. In order for this to occur the subset of grains must possess some advantage over their competitors such as a high grain boundary energy, locally high grain boundary mobility, favourable texture or lower local second-phase particle density.
Factors hindering growth
If there are additional factors preventing boundary movement, such as Zener pinningZener pinning
Zener pinning is the influence of a dispersion of fine particles on the movement of low- and high angle grain boundaries through a polycrystalline material. Small particles act to prevent the motion of such boundaries by exerting a pinning pressure which counteracts the driving force pushing the...
by particles, then the grain size may be restricted to a much lower value than might otherwise be expected. This is an important industrial mechanism in preventing the softening of materials at high temperature.

