
Globin fold
Encyclopedia
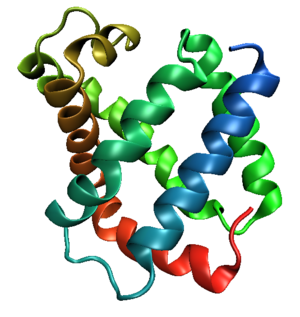
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s. This fold typically consists of eight alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
, although some proteins have additional helix extensions at their termini. The globin fold is found in its namesake proteins hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
and myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
as well as in phycocyanin
Phycocyanin
Phycocyanin is a pigment from the light-harvesting phycobiliprotein family, along with allophycocyanin and phycoerythrin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble and therefore cannot exist within the membrane as do carotenoids, but aggregate forming...
proteins. Because myoglobin was the first protein whose structure was solved, the globin fold was thus the first protein fold discovered. Since the globin fold contains only helices, it is classified as an all-alpha protein fold.
Helix packing
The eight helices of the globin fold core share significant nonlocal structure, unlike other structural motifStructural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
s in which amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s close to each other in primary sequence are also close in space. The helices pack together at an average angle of about 50 degrees, significantly steeper than other helical packings such as the helix bundle
Helix bundle
A helix bundle is a small protein fold composed of several alpha helices that are usually nearly parallel or antiparallel to each other.-Three-helix bundles:Three-helix bundles are among the smallest and fastest known cooperatively folding structural domains...
. The exact angle of helix packing depends on the sequence of the protein, because packing is mediated by the sterics and hydrophobic interactions of the amino acid side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
s near the helix interfaces.
Sequence conservation
Although the globin fold is highly evolutionEvolution
Evolution is any change across successive generations in the heritable characteristics of biological populations. Evolutionary processes give rise to diversity at every level of biological organisation, including species, individual organisms and molecules such as DNA and proteins.Life on Earth...
arily conserved
Conserved sequence
In biology, conserved sequences are similar or identical sequences that occur within nucleic acid sequences , protein sequences, protein structures or polymeric carbohydrates across species or within different molecules produced by the same organism...
, the sequences that form the fold can have as low as 16% sequence identity. While the sequence specificity of the fold is not stringent, the hydrophobic core of the protein must be maintained and hydrophobic patches on the generally hydrophilic solvent-exposed surface must be avoided in order for the structure to remain stable and soluble. The most famous mutation in the globin fold is a change from glutamate to valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
in one chain of the hemoglobin molecule. This mutation creates a "hydrophobic patch" on the protein surface that promotes intermolecular aggregation, the molecular event that gives rise to sickle-cell anemia.

