
Geiger–Müller tube
Encyclopedia
Geiger counter
A Geiger counter, also called a Geiger–Müller counter, is a type of particle detector that measures ionizing radiation. They detect the emission of nuclear radiation: alpha particles, beta particles or gamma rays. A Geiger counter detects radiation by ionization produced in a low-pressure gas in a...
instrument that can detect a single particle of ionizing radiation
Ionizing radiation
Ionizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
, and typically produce an audible click for each. It was named for Hans Geiger who invented the device in 1908, and Walther Müller
Walther Müller
Walther Müller , was a German physicist, most well known for his improvement of Hans Geiger's counter for ionizing radiation, now known as the Geiger-Müller tube....
who collaborated with Geiger in developing it further in 1928. It is a type of gaseous ionization detector.
The Geiger counter is sometimes used as a hardware random number generator
Hardware random number generator
In computing, a hardware random number generator is an apparatus that generates random numbers from a physical process. Such devices are often based on microscopic phenomena that generate a low-level, statistically random "noise" signal, such as thermal noise or the photoelectric effect or other...
.
Description and operation
A Geiger–Müller tube consists of a tubeGas filled tube
A gas-filled tube, also known as a discharge tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Although the envelope is typically glass, power tubes often use ceramics, and military tubes often use glass-lined metal...
filled with a low-pressure inert gas such as helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
or argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
(usually neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
), in some cases in a Penning mixture
Penning mixture
A Penning mixture , named after Frans Michel Penning, is a mixture of gases used in electric lighting or displaying fixtures. Although the popular phrase for the most common of these is a neon lamp, it's more efficient to have the glass tube filled not with pure neon, but with a Penning mixture,...
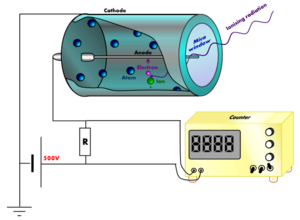
, and an organic vapor or a halogen gas. The tube contains electrodes, between which there is a potential difference of several hundred volt
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s, but no current flowing. The walls of the tube are either entirely metal or have their inside surface coated with a conductor to form the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
while the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
is a wire
Wire
A wire is a single, usually cylindrical, flexible strand or rod of metal. Wires are used to bear mechanical loads and to carry electricity and telecommunications signals. Wire is commonly formed by drawing the metal through a hole in a die or draw plate. Standard sizes are determined by various...
passing up the center of the tube.
When ionizing radiation
Ionizing radiation
Ionizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
passes through the tube, some of the gas molecules are ionized, creating positively charged ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s, and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s. The strong electric field created by the tube's electrodes accelerates the ions towards the cathode and the electrons towards the anode. The ion pairs gain sufficient energy to ionize further gas molecules through collisions on the way, creating an avalanche
Electron avalanche
An electron avalanche is a process in which a number of free electrons in a medium are subjected to strong acceleration by an electric field, ionizing the medium's atoms by collision , thereby forming "new" electrons to undergo the same process in successive cycles...
of charged particles.
This results in a short, intense pulse of current which passes (or cascades) from the negative electrode to the positive electrode and is measured or counted.
Most detectors include an audio amplifier
Audio amplifier
An audio amplifier is an electronic amplifier that amplifies low-power audio signals to a level suitable for driving loudspeakers and is the final stage in a typical audio playback chain.The preceding stages in such a chain are low power audio amplifiers which perform tasks like pre-amplification,...
that produce an audible click on discharge. The number of pulses per second
Second
The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock....
measures the intensity of the radiation field. Some Geiger counters display an exposure rate (e.g. mR/h
Röntgen
The roentgen is a unit of measurement for exposure to ionizing radiation , and is named after the German physicist Wilhelm Röntgen...
), but this does not relate easily to a dose rate
Absorbed dose
Absorbed dose is a measure of the energy deposited in a medium by ionizing radiation per unit mass...
as the instrument does not discriminate between radiation of different energies.
Geiger plateau
The Geiger plateau is the voltage range in which the Geiger–Müller counter operates. If a GM tube is exposed to a steady radiation source and the applied voltage increased from zero, at first the count rate increases rapidly; at a certain voltage the rate of increase flattens out (only changing a few per cent for every 100 volts increase).Depending on the characteristics of the specific tube (manufacturer, size, gas type etc.) the exact voltage range may vary. In this plateau region, the potential difference in the counter is strong enough to ionize
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
all the gas inside the tube, upon triggering by the incoming ionizing radiation (alpha, beta or gamma radiation). Below the plateau the voltage is not high enough to cause complete discharge; a limited Townsend avalanche is the result, and the tube acts as a proportional counter
Proportional counter
A proportional counter is a measurement device to count particles of ionizing radiation and measure their energy.A proportional counter is a type of gaseous ionization detector. Its operation is similar to that of a Geiger-Müller counter, but uses a lower operating voltage. An inert gas is used to...
, where the output pulse size depends on the initial ionization created by the radiation. Higher voltages give a pulse size independent of the initial ionization energy. If the applied voltage is too high, a continuous glow discharge is formed and the tube cannot detect radiation.
The plateau has a slight incline caused by increased sensitivity to low energy radiation, due to the increased voltage on the device. Normally when a particle enters the tube and ionizes one of the gas atoms, complete ionization of the gas occurs. Once a low energy particle enters the counter, it is possible that the kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
in addition to the potential energy of the voltage are insufficient for the additional ionization to occur and thus the ion recombines. At higher voltages, the threshold for the minimum radiation level drops, thus the counter's sensitivity rises. The counting rate for a given radiation source varies slightly as the applied voltage is varied; for standardization of the response of the instrument, a regulated voltage is used to maintain stable counting characteristics.
GM tubes
The usual form of GM tube is an end-window tube. This type is so-named because the tube has a window at one end through which ionizing radiation can easily penetrate. The other end normally has the electrical connectors. There are two types of end-window tubes: the glassGlass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
-mantle type and the mica
Mica
The mica group of sheet silicate minerals includes several closely related materials having highly perfect basal cleavage. All are monoclinic, with a tendency towards pseudohexagonal crystals, and are similar in chemical composition...
window type. The glass window type will not detect alpha radiation
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
since it is unable to penetrate the glass, but is usually cheaper and will usually detect beta radiation and X-rays. The mica window type will detect alpha radiation but is more fragile.
Most tubes will detect gamma radiation, and usually beta radiation above about 2.5 MeV
MEV
MeV and meV are multiples and submultiples of the electron volt unit referring to 1,000,000 eV and 0.001 eV, respectively.Mev or MEV may refer to:In entertainment:* Musica Elettronica Viva, an Italian musical group...
. Geiger–Müller tubes will not normally detect neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s since these do not ionise the gas. However, neutron-sensitive tubes can be produced which either have the inside of the tube coated with boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
or contain boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
or helium-3
Helium-3
Helium-3 is a light, non-radioactive isotope of helium with two protons and one neutron. It is rare on Earth, and is sought for use in nuclear fusion research...
gas. The neutrons interact with the boron nuclei, producing alpha particles or with the helium-3 nuclei producing hydrogen and tritium
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
ions and electrons. These charged particles then trigger the normal avalanche process.
Although most tubes will detect gamma radiation, standard tubes are relatively inefficient, as most gamma photons will pass through the low density gas without interacting. Using the heavier noble gases
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
or xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
for the fill effects a small improvement, but dedicated gamma detectors use dense cathodes of lead or stainless steel in windowless tubes. The dense cathode then interacts with the gamma flux, producing high-energy electrons, which are then detected.
Quenching and dead time
The ideal GM tube should produce a single pulse on entry of a single ionising particle. It must not give any spurious pulses, and must recover quickly to the passive state. Unfortunately for these requirements, when positive argon ions reach the cathode and become neutral argon atoms again by obtaining electrons from it, the atoms can acquire their electrons in enhanced energy levels. These atoms then return to their ground state by emitting photons which can in turn produce further ionisation and hence cause spurious secondary pulse discharges. If nothing were done to counteract it, ionisation could even escalate, causing a so-called current "avalanche" which if prolonged could damage the tube. Some form of quenching of the ionisation is therefore essential. The disadvantage of quenching is that for a short time after a discharge pulse has occurred (the so-called dead time, which is typically a few microseconds), the tube is rendered insensitive and is thus temporarily unable to detect the arrival of any new ionising particle. This effectively causes a loss of counts at sufficiently-high count rates.External quenching uses control electronics to temporarily remove the high voltage between the electrodes. Self-quenching or internal-quenching tubes stop the discharge without external assistance, by means of the addition of a small amount of a polyatomic organic vapor such as butane or ethanol; or alternatively a halogen such as bromine or chlorine.
If a poor diatomic gas quencher were introduced to the tube, the positive argon ions, during their motion toward the cathode, would have multiple collisions with the quencher gas molecules and transfer their charge and some energy to them. Neutral argon atoms would then be produced and the quencher gas ions would reach the cathode instead, gain electrons in excited states which would decay by photon emission, thereby producing spurious tube discharge as before. However, effective quencher molecules, when excited, do not lose their energy by photon emission but by dissociation into neutral quencher atoms. No spurious output pulses are then produced.
Halogen tube
The halogen GM tube was invented by Sidney H. LiebsonSidney H. Liebson
Sidney H. Liebson received his Ph.D. from the University of Maryland in 1947. His thesis was on the discharge mechanism of Geiger Muller counters...
in 1947, The discharge mechanism takes advantage of a metastable state of the inert gas atom to more-readily ionize a halogen molecule, enabling the tube to operate at much lower voltages, typically 400–600 volts instead of 900–1200 volts. This type of GM tube is therefore by far the most common form now. It has a longer life than tubes quenched with organic compounds, because the halogen ions can recombine while the organic vapor is gradually destroyed by the discharge process (giving the latter a life of around 108 events)
See also
- DosimeterDosimeterDosimeters measure an individual's or an object'sexposure to something in the environment — particularly to a hazard inflicting cumulative impact over long periods of time, or over a lifetime...
- Geiger counterGeiger counterA Geiger counter, also called a Geiger–Müller counter, is a type of particle detector that measures ionizing radiation. They detect the emission of nuclear radiation: alpha particles, beta particles or gamma rays. A Geiger counter detects radiation by ionization produced in a low-pressure gas in a...
- Gaseous ionization detectorsGaseous ionization detectorsIn particle physics, gaseous ionization detectors are detectors designed to seek the presence of particles . If a particle has enough energy to ionize a gas atom or molecule, the resulting electrons and ions cause a current flow which can be measured in different ways...
- Ionization chamberIonization chamberThe ionization chamber is the simplest of all gas-filled radiation detectors, and is used for the detection or measurement of ionizing radiation...
External links
Patents, H. J. Spanner, "Gas Filled Tube", G. J. Weissenberg, "Electron Discharge Tube", J. A. Victoreen, "Geiger tube", J. A. Victoreen, "Geiger tube"Other

