
Galileo thermometer
Encyclopedia

Galileo Galilei
Galileo Galilei , was an Italian physicist, mathematician, astronomer, and philosopher who played a major role in the Scientific Revolution. His achievements include improvements to the telescope and consequent astronomical observations and support for Copernicanism...
, is a thermometer
Thermometer
Developed during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
made of a sealed glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
cylinder
Cylinder (geometry)
A cylinder is one of the most basic curvilinear geometric shapes, the surface formed by the points at a fixed distance from a given line segment, the axis of the cylinder. The solid enclosed by this surface and by two planes perpendicular to the axis is also called a cylinder...
containing a clear liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and a series of objects whose densities are such that they rise or fall as the temperature changes. By definition, Galileo's thermometer is actually a thermoscope, not a thermometer.
Normal design
Suspended in the cocoare a number of weights. Commonly those weights are themselves attached to sealed glass bulbs containing colored liquid for aesthetic effect. As the liquid in the cylinder changes temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, its density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
changes and the bulbs are free to move – rising or falling to reach a position where their density is either equal to that of the surrounding liquid or where they are brought to a halt by other bulbs. If the bulbs differ in density by a very small amount and are ordered such that the least dense is at the top and densest at the bottom, they can form a temperature scale.
The temperature is typically read from an engraved metal disc on each bulb. Usually a gap separates the top bulbs from the bottom bulbs and then the temperature would be between the tag readings on either side of the gap. If a bulb is free-floating in the gap, then its tag reading would be closest to the ambient temperature.
To achieve satisfactory accuracy, the weights are required to be manufactured to a tolerance of less than 1/1000 of one gram (1 milligram (3.5273962105112E-05 oz)).
Theory of operation

Buoyancy
In physics, buoyancy is a force exerted by a fluid that opposes an object's weight. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus a column of fluid, or an object submerged in the fluid, experiences greater pressure at the bottom of the...
. Buoyancy determines whether objects float or sink in a liquid, and is responsible for the fact that even boats made of steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
can float (while a solid bar of steel by itself will sink).
The only factor that determines whether a large object will rise or fall in a particular liquid relates the object's density to the density of the liquid in which it is placed. If the object's mass is greater than the mass of liquid displaced, the object will sink. If the object's mass is less than the mass of liquid displaced, the object will float.
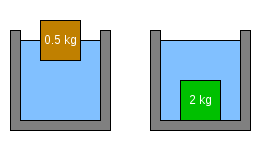

In the examples above, the liquid in which the objects have been floating is assumed to be water. Water has a density of 1 kg/L, which means that the mass of water displaced by any of the above objects when fully submerged, is 1 kg (2.2 lb).
Galileo discovered that the density of a liquid is a function of its temperature. This is the key to how the Galileo thermometer works; as the temperature of water increases from 4 °C (39.2 °F), its density decreases.

In the Galileo thermometer, the small glass bulbs are partly filled with a different (coloured) liquid. Once the handblown
Glassblowing
Glassblowing is a glassforming technique that involves inflating molten glass into a bubble, or parison, with the aid of a blowpipe, or blow tube...
bulbs have been sealed, their effective densities are adjusted by means of the metal tags hanging from beneath them. The expansion due to the temperature change of the coloured liquid and air gap (called ullage
Ullage
Ullage or Headspace refers to the unfilled space in a container, particularly with a liquid.-Etymology:The word comes ultimately from the Latin oculus, “eye”, which was used in a figurative sense by the Romans for the bung hole of a barrel...
) inside the bulbs would affect their density were it not for the almost fixed volume of the glass bulb. The clear liquid in which the bulbs are submerged is not water, but some inert hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
whose density varies with temperature more than water's density does. This change of density of the clear liquid, with temperature change, causes the bulbs to rise or sink.
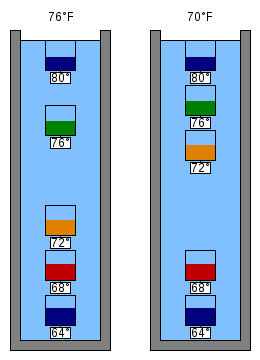
If there are some bulbs at the top (Figure 4, left) and some at the bottom, but one floating in the gap, then the one floating in the gap (green 76 °F (24.4 °C)) tells the temperature. If there is no bulb in the gap (Figure 4, right) then the average of the values of the bulb above and below the gap gives the approximate temperature.
The bulbs and weights should be sized so as not to jam with each other, either by being at least half the size of the tube diameter to retain their stacking order or, as an alternative, much less than the tube diameter to freely pass each other in the tubes.

