
Furanose
Encyclopedia
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
, but the furanose ring does not have double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s.
Structural properties
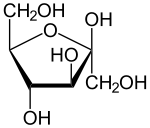
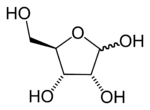
The furanose ring is a cyclic hemiacetalHemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
of an aldopentose or a cyclic hemiketal of a ketohexose
Ketohexose
A ketohexose is a ketone-containing hexose . The most common ketohexoses, each of which represents a pair of enantiomers , include fructose, psicose, sorbose, and tagatose...
.
A furanose ring structure consists of four carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and one oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom with the anomer
Anomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
ic carbon to the right of the oxygen. The highest numbered chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
carbon (typically to the left of the oxygen in a Haworth projection
Haworth projection
A Haworth projection is a common way of representing the cyclic structure of monosaccharides with a simple three-dimensional perspective.The Haworth projection was named after the English chemist Sir Norman Haworth....
) determines whether or not the structure has a -configuration or L-configuration. In an -configuration furanose, the substituent on the highest numbered chiral carbon is pointed downwards out of the plane, and in a D-configuration furanose, the highest numbered chiral carbon is facing upwards.
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group is pointing. In a -configuration furanose, alpha configuration has the hydroxy pointing down, and beta has the hydroxy pointing up. It is the opposite in an -configuration furanose. Typically, the anomeric carbon undergoes mutarotation
Mutarotation
Mutarotation is the change in the optical rotation that occurs by epimerization...
in solution, and the result is an equilibrium mixture of alpha-beta configurations.

