Franck-Hertz experiment
Overview
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
. In 1914, the German physicists James Franck
James Franck
James Franck was a German Jewish physicist and Nobel laureate.-Biography:Franck was born to Jacob Franck and Rebecca Nachum Drucker. Franck completed his Ph.D...
and Gustav Ludwig Hertz
Gustav Ludwig Hertz
Gustav Ludwig Hertz was a German experimental physicist and Nobel Prize winner, and a nephew of Heinrich Rudolf Hertz.-Biography:...
sought to experimentally probe the energy levels of the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
. The now-famous Franck–Hertz experiment elegantly supported Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
's model of the atom
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
, with electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s orbiting the nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
with specific, discrete energies. Franck and Hertz were awarded the Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
in 1925 for this work.
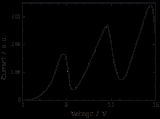
The Franck–Hertz experiment confirmed Bohr's quantized model of the atom by demonstrating that atoms could indeed only absorb (and be excited by) specific amounts of energy (quanta).
The classic experiment involved a tube containing low pressure gas fitted with three electrodes: an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
-emitting cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, a mesh grid
Control grid
The control grid is an electrode used in thermionic valves used to modulate the flow of electrons in the cathode to anode or plate circuit.- Operation :...
for acceleration, and an anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
.
Unanswered Questions

