
Fractionating column
Encyclopedia
A fractionating column or fractionation column is an essential item used in the distillation
of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities. Fractionating columns are used in small scale laboratory distillations as well as for large-scale industrial distillations.
s.
 Fractionating columns help to separate the mixture by allowing the mixed vapors to cool, condense
Fractionating columns help to separate the mixture by allowing the mixed vapors to cool, condense
, and vaporize again in accordance with Raoult's law. With each condensation
-vaporization cycle, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. This is the rationale for a Vigreux fractionating column or a packed fractionating column. Spinning band distillation
achieves the same outcome by using a rotating band within the column to force the rising vapors and descending condensate into close contact, achieving equilibrium more quickly.
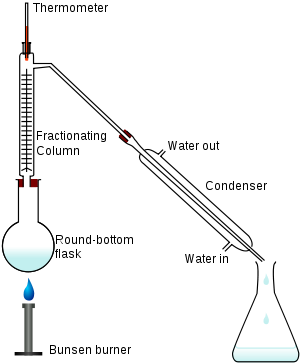
In a typical fractional distillation, a liquid mixture is heated in the distilling flask, and the resulting vapor rises up the fractionating column (see Figure 1). The vapor condenses on glass spurs (known as trays or plates
) inside the column, and returns to the distilling flask, reflux
ing the rising distillate vapor. The hottest tray is at the bottom of the column and the coolest tray is at the top. At steady-state conditions, the vapor and liquid on each tray reach an equilibrium
. Only the most volatile of the vapors stays in gas form all the way to the top, where it may then proceed through a condenser
, which cools the vapor until it condenses into a liquid distillate. The separation may be enhanced by the addition of more trays (to a practical limitation of heat, flow, etc.).

is one of the unit operations of chemical engineering
. Fractionating columns are widely used in the chemical process industries where large quantities of liquids have to be distilled. Such industries are the petroleum
processing, petrochemical
production, natural gas processing
, coal tar
processing, brewing
, liquified air
separation, and hydrocarbon
solvents production and similar industriesб but it finds its widest application in petroleum refineries
. In such refineries, the crude oil feedstock is a complex, multicomponent mixture that must be separated, and yields of pure chemical compounds are not expected, only groups of compounds within a relatively small range of boiling points
, also called fractions. That is the origin of the name fractional distillation or fractionation. It is often not worthwhile separating the components in these fractions any further based on product requirements and economics.
Distillation is one of the most common and energy-intensive separation processes. In a typical chemical plant, it accounts for about 40% of the total energy consumption. Industrial distillation is typically performed in large, vertical cylindrical columns (as shown in Figure 2) known as "distillation towers" or "distillation columns" with diameters ranging from about 65 centimeters to 6 meters and heights ranging from about 6 meters to 60 meters or more.
 Industrial distillation towers are usually operated at a continuous steady state. Unless disturbed by changes in feed, heat, ambient temperature, or condensing, the amount of feed being added normally equals the amount of product being removed.
Industrial distillation towers are usually operated at a continuous steady state. Unless disturbed by changes in feed, heat, ambient temperature, or condensing, the amount of feed being added normally equals the amount of product being removed.
It should also be noted that the amount of heat entering the column from the reboiler
and with the feed must equal the amount heat removed by the overhead condenser and with the products. The heat entering a distillation column is a crucial operating parameter, addition of excess or insufficient heat to the column can lead to foaming, weeping, entrainment, or flooding.
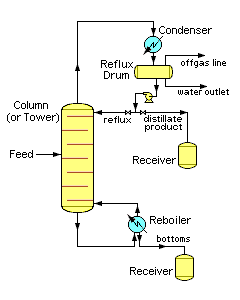
Figure 3 depicts an industrial fractionating column separating a feed stream into one distillate fraction and one bottoms fraction. However, many industrial fractionating columns have outlets at intervals up the column so that multiple products having different boiling ranges may be withdrawn from a column distilling a multi-component feed stream. The "lightest" products with the lowest boiling points exit from the top of the columns and the "heaviest" products with the highest boiling points exit from the bottom.
Industrial fractionating columns use external reflux to achieve better separation of products. Reflux refers to the portion of the condensed overhead liquid product that returns to the upper part of the fractionating column as shown in Figure 3.
Inside the column, the downflowing reflux liquid provides cooling and condensation of upflowing vapors thereby increasing the efficacy of the distillation tower. The more reflux and/or more trays provided, the better is the tower's separation of lower boiling materials from higher boiling materials.
The design and operation of a fractionating column depends on the composition of the feed and as well as the composition of the desired products. Given a simple, binary component feed, analytical methods such as the McCabe-Thiele method
or the Fenske equation
can be used. For a multi-component feed, simulation models are used both for design, operation, and construction.
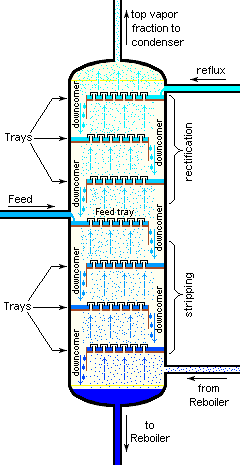
Bubble-cap "trays" or "plates" are one of the types of physical devices, which are used to provide good contact between the upflowing vapor and the downflowing liquid inside an industrial fractionating column. Such trays are shown in Figures 4 and 5.
The efficiency of a tray or plate is typically lower than that of a theoretical 100% efficient equilibrium stage. Hence, a fractionating column almost always needs more actual, physical plates than the required number of theoretical vapor-liquid equilibrium
stages.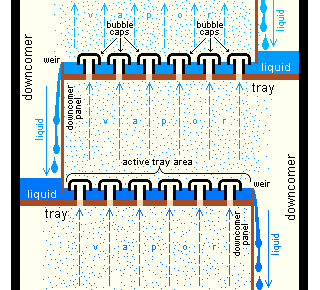 In industrial uses, sometimes a packing material
In industrial uses, sometimes a packing material
is used in the column instead of trays, especially when low pressure drops across the column are required, as when operating under vacuum
. This packing material can either be random dumped packing (1–3" wide) such as Raschig ring
s or structured sheet metal
. Liquids tend to wet the surface of the packing, and the vapors pass across this wetted surface, where mass transfer
takes place. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities. Fractionating columns are used in small scale laboratory distillations as well as for large-scale industrial distillations.
Laboratory fractionating columns
A laboratory fractionating column is a piece of glassware used to separate vaporized mixtures of liquid compounds with close voltality. It can also be called a fractional column. Most commonly used is either a Vigreux column or a straight column packed with glass beads or metal pieces such as Raschig ringRaschig ring
Raschig rings are pieces of tube used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic or metal and provide a large surface area within the volume of the column for interaction between liquid and gas or vapour...
s.

Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
, and vaporize again in accordance with Raoult's law. With each condensation
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
-vaporization cycle, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. This is the rationale for a Vigreux fractionating column or a packed fractionating column. Spinning band distillation
Spinning band distillation
Spinning band distillation is a technique used to separate liquid mixtures which are similar in boiling points. When liquids with similar boiling points are distilled, the vapors are mixtures, and not pure compounds. Fractionating columns help separate the mixture by allowing the mixed vapors to...
achieves the same outcome by using a rotating band within the column to force the rising vapors and descending condensate into close contact, achieving equilibrium more quickly.
In a typical fractional distillation, a liquid mixture is heated in the distilling flask, and the resulting vapor rises up the fractionating column (see Figure 1). The vapor condenses on glass spurs (known as trays or plates
Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
) inside the column, and returns to the distilling flask, reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
ing the rising distillate vapor. The hottest tray is at the bottom of the column and the coolest tray is at the top. At steady-state conditions, the vapor and liquid on each tray reach an equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
. Only the most volatile of the vapors stays in gas form all the way to the top, where it may then proceed through a condenser
Condenser (heat transfer)
In systems involving heat transfer, a condenser is a device or unit used to condense a substance from its gaseous to its liquid state, typically by cooling it. In so doing, the latent heat is given up by the substance, and will transfer to the condenser coolant...
, which cools the vapor until it condenses into a liquid distillate. The separation may be enhanced by the addition of more trays (to a practical limitation of heat, flow, etc.).

Industrial fractionating columns
Fractional distillationFractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
is one of the unit operations of chemical engineering
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
. Fractionating columns are widely used in the chemical process industries where large quantities of liquids have to be distilled. Such industries are the petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
processing, petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
production, natural gas processing
Natural gas processing
Natural-gas processing is a complex industrial process designed to clean raw natural gas by separating impurities and various non-methane hydrocarbons and fluids to produce what is known as pipeline quality dry natural gas.-Background:...
, coal tar
Coal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
processing, brewing
Brewing
Brewing is the production of beer through steeping a starch source in water and then fermenting with yeast. Brewing has taken place since around the 6th millennium BCE, and archeological evidence suggests that this technique was used in ancient Egypt...
, liquified air
Liquid air
Liquid air is air that has been cooled to very low temperatures so that it has condensed to a pale blue mobile liquid. To protect it from room temperature, it must be kept in a vacuum flask. Liquid air can absorb heat rapidly and revert to its gaseous state...
separation, and hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
solvents production and similar industriesб but it finds its widest application in petroleum refineries
Oil refinery
An oil refinery or petroleum refinery is an industrial process plant where crude oil is processed and refined into more useful petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas...
. In such refineries, the crude oil feedstock is a complex, multicomponent mixture that must be separated, and yields of pure chemical compounds are not expected, only groups of compounds within a relatively small range of boiling points
Boiling Points
Boiling Points is a prank reality television show, much like the format used on Candid Camera. It is broadcast on MTV in the United States. In each half-hour episode, annoying situations are set up and deliberately inflicted on one or more young adults who are unaware that they are being tested...
, also called fractions. That is the origin of the name fractional distillation or fractionation. It is often not worthwhile separating the components in these fractions any further based on product requirements and economics.
Distillation is one of the most common and energy-intensive separation processes. In a typical chemical plant, it accounts for about 40% of the total energy consumption. Industrial distillation is typically performed in large, vertical cylindrical columns (as shown in Figure 2) known as "distillation towers" or "distillation columns" with diameters ranging from about 65 centimeters to 6 meters and heights ranging from about 6 meters to 60 meters or more.


It should also be noted that the amount of heat entering the column from the reboiler
Reboiler
Reboilers are heat exchangers typically used to provide heat to the bottom of industrial distillation columns. They boil the liquid from the bottom of a distillation column to generate vapors which are returned to the column to drive the distillation separation....
and with the feed must equal the amount heat removed by the overhead condenser and with the products. The heat entering a distillation column is a crucial operating parameter, addition of excess or insufficient heat to the column can lead to foaming, weeping, entrainment, or flooding.
Figure 3 depicts an industrial fractionating column separating a feed stream into one distillate fraction and one bottoms fraction. However, many industrial fractionating columns have outlets at intervals up the column so that multiple products having different boiling ranges may be withdrawn from a column distilling a multi-component feed stream. The "lightest" products with the lowest boiling points exit from the top of the columns and the "heaviest" products with the highest boiling points exit from the bottom.
Industrial fractionating columns use external reflux to achieve better separation of products. Reflux refers to the portion of the condensed overhead liquid product that returns to the upper part of the fractionating column as shown in Figure 3.
Inside the column, the downflowing reflux liquid provides cooling and condensation of upflowing vapors thereby increasing the efficacy of the distillation tower. The more reflux and/or more trays provided, the better is the tower's separation of lower boiling materials from higher boiling materials.
The design and operation of a fractionating column depends on the composition of the feed and as well as the composition of the desired products. Given a simple, binary component feed, analytical methods such as the McCabe-Thiele method
McCabe-Thiele method
The McCabe-Thiele method was presented by two graduate students at Massachusetts Institute of Technology , Warren L. McCabe and Ernest W. Thiele in 1925. The technique is considered to be the simplest and perhaps most instructive method for analysis of binary distillation...
or the Fenske equation
Fenske equation
The Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux .The equation was derived by Merrell Fenske in...
can be used. For a multi-component feed, simulation models are used both for design, operation, and construction.
Bubble-cap "trays" or "plates" are one of the types of physical devices, which are used to provide good contact between the upflowing vapor and the downflowing liquid inside an industrial fractionating column. Such trays are shown in Figures 4 and 5.
The efficiency of a tray or plate is typically lower than that of a theoretical 100% efficient equilibrium stage. Hence, a fractionating column almost always needs more actual, physical plates than the required number of theoretical vapor-liquid equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
stages.

Packed bed
In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packing can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing...
is used in the column instead of trays, especially when low pressure drops across the column are required, as when operating under vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
. This packing material can either be random dumped packing (1–3" wide) such as Raschig ring
Raschig ring
Raschig rings are pieces of tube used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic or metal and provide a large surface area within the volume of the column for interaction between liquid and gas or vapour...
s or structured sheet metal
Structured packing
The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns and chemical reactors...
. Liquids tend to wet the surface of the packing, and the vapors pass across this wetted surface, where mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
takes place. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
See also
- Azeotropic distillationAzeotropic distillationIn chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, azeotropic distillation usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous...
- Batch distillationBatch distillationBatch distillation refers to the use of distillation in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated...
- Continuous distillationContinuous distillationContinuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation or partial separation of a liquid feed mixture into components or...
- Extractive distillationExtractive distillationExtractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity...
- Laboratory glasswareLaboratory glasswareLaboratory glassware refers to a variety of equipment, traditionally made of glass, used for scientific experiments and other work in science, especially in chemistry and biology laboratories...
- Steam distillationSteam distillationSteam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds....
- Theoretical plateTheoretical plateA theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
- Vacuum distillationVacuum distillationVacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure causing evaporation of the most volatile liquid...
External links
- Use of distillation columns in Oil & Gas
- More drawings of glassware including Vigreux columns
- Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad, Norwegian University of Science and Technology, Norway
- Distillation, An Introduction by Ming Tham, Newcastle University, UK
- Distillation by the Distillation Group, USA
- Distillation simulation software
- Fractional Distillation Explained for High School Students

