
FLiNaK
Encyclopedia
FLiNaK is the name of the ternary eutectic alkaline metal fluoride
salt
mixture LiF
-NaF
-KF
(46.5-11.5-42 mol %). It has a melting point of 454 °C and a boiling point of 1570 °C. It is used as electrolyte
for the electroplating
of refractory metals
and compounds like titanium
, tantalum
, hafnium
, zirconium
and their boride
s. FLiNaK also could see potential use as a coolant in the Very High Temperature Reactor
, a type of nuclear reactor.
as potential candidate for a coolant in the Molten Salt Reactor
because of its low melting point, high heat capacity, and its chemical stability at high temperatures. Ultimately, its sister salt, FLiBe
, was chosen as the solvent salt for the molten salt reactor due to a more desirable nuclear cross section. FLiNaK still gathers interest as a intermediate coolant for a high temperature molten salt reactor where it could transfer heat without being in the presence of the fuel.
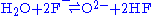
All of these reactions work together to create hydrofluoric acid
which is highly corrosive to many metals. All of these reactions can be reversed by adding a combination of hydrogen gas and hydrofluoric to the molten salt yielding water, which can be boiled off. Theoretically, if contaminates could be taken out of the salt, no corrosion would occur. This process is accelerated by the high temperatures encountered in a nuclear reactor and is one of the main problems in a molten salt reactor. Some compounds, such as the nickel-based Hastelloy-N were engineered specifically for the molten salt reactor experiment and proved highly resistant to corrosion by molten fluoride salts.
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
mixture LiF
Lithium fluoride
Lithium fluoride is an inorganic compound with the formula LiF. It is the lithium salt of hydrofluoric acid. This white solid is a simple ionic compound. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten...
-NaF
Sodium fluoride
Sodium fluoride is an inorganic chemical compound with the formula NaF. A colorless solid, it is a source of the fluoride ion in diverse applications. Sodium fluoride is less expensive and less hygroscopic than the related salt potassium fluoride....
-KF
Potassium fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali metal halide and occurs naturally as the rare mineral carobbiite...
(46.5-11.5-42 mol %). It has a melting point of 454 °C and a boiling point of 1570 °C. It is used as electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
for the electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
of refractory metals
Refractory metals
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definition of which elements belong to this group differs...
and compounds like titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
, hafnium
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable...
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and their boride
Boride
In chemistry a boride is a chemical compound between boron and a less electronegative element, for example silicon boride . The borides are a very large group of compounds that are generally high melting and are not ionic in nature. Some borides exhibit very useful physical properties. The term...
s. FLiNaK also could see potential use as a coolant in the Very High Temperature Reactor
Very high temperature reactor
The Very High Temperature Reactor , or High Temperature Gas-cooled Reactor , is a Generation IV reactor concept that uses a graphite-moderated nuclear reactor with a once-through uranium fuel cycle. The VHTR is a type of High Temperature Reactor that can conceptually have an outlet temperature of...
, a type of nuclear reactor.
Coolant
FLiNaK salt was researched heavily during the late 1950s by Oak Ridge National LaboratoryOak Ridge National Laboratory
Oak Ridge National Laboratory is a multiprogram science and technology national laboratory managed for the United States Department of Energy by UT-Battelle. ORNL is the DOE's largest science and energy laboratory. ORNL is located in Oak Ridge, Tennessee, near Knoxville...
as potential candidate for a coolant in the Molten Salt Reactor
Molten salt reactor
A molten salt reactor is a type of nuclear fission reactor in which the primary coolant, or even the fuel itself is a molten salt mixture...
because of its low melting point, high heat capacity, and its chemical stability at high temperatures. Ultimately, its sister salt, FLiBe
FLiBe
FLiBe is a mixture of lithium fluoride and beryllium fluoride . As a molten salt it is proposed as a nuclear reactor coolant, and two different mixtures were used in the Molten-Salt Reactor Experiment....
, was chosen as the solvent salt for the molten salt reactor due to a more desirable nuclear cross section. FLiNaK still gathers interest as a intermediate coolant for a high temperature molten salt reactor where it could transfer heat without being in the presence of the fuel.
Corrosion
Fluoride salts, like all salts, cause corrosion in most metals and alloys. There are three mechanism through which they can cause corrosion: impurities in the salt, temperature gradients in the salt system, and chemical activity gradients. O2, H20, and all oxides are the main impurities that cause corrosion through the following reactions: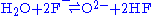
- Water and fluoride ions create acid and oxygen.

- Water and fluoride ions create acid and hydroxide.
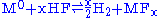
- Acid oxidizes M, where M is any suitable metal (ex: Cr, Fe, Ni).
All of these reactions work together to create hydrofluoric acid
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
which is highly corrosive to many metals. All of these reactions can be reversed by adding a combination of hydrogen gas and hydrofluoric to the molten salt yielding water, which can be boiled off. Theoretically, if contaminates could be taken out of the salt, no corrosion would occur. This process is accelerated by the high temperatures encountered in a nuclear reactor and is one of the main problems in a molten salt reactor. Some compounds, such as the nickel-based Hastelloy-N were engineered specifically for the molten salt reactor experiment and proved highly resistant to corrosion by molten fluoride salts.

