
Exergonic reaction
Encyclopedia
An exergonic reaction is a chemical reaction
where the change in the Gibbs free energy
is negative, indicating a spontaneous reaction. Symbolically, the release of Gibbs free energy, G, in an exergonic reaction is denoted as
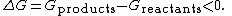
Although exergonic reactions are said to occur spontaneously, this does not imply that the reaction will take place at an observable rate
. For instance, the disproportionation of hydrogen peroxide is very slow in the absence of a suitable catalyst. It has been suggested that eager would be a more intuitive term in this context.
More generally, the terms exergonic
and endergonic
relate to the Gibbs free energy
change in any process, not just chemical reactions. An example of an exergonic reaction is cellular respiration.
The terms exothermic
and endothermic
reactions relate to the enthalpy
change of a process.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
where the change in the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
is negative, indicating a spontaneous reaction. Symbolically, the release of Gibbs free energy, G, in an exergonic reaction is denoted as
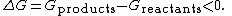
Although exergonic reactions are said to occur spontaneously, this does not imply that the reaction will take place at an observable rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
. For instance, the disproportionation of hydrogen peroxide is very slow in the absence of a suitable catalyst. It has been suggested that eager would be a more intuitive term in this context.
More generally, the terms exergonic
Exergonic
Exergonic means "releasing energy in the form of work". By thermodynamic standards, work, a form of energy, is defined as moving from the system to the surroundings...
and endergonic
Endergonic
Endergonic means "absorbing energy in the form of work." Endergonic reactions are not spontaneous...
relate to the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
change in any process, not just chemical reactions. An example of an exergonic reaction is cellular respiration.
The terms exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
and endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
reactions relate to the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change of a process.

