
Excimer
Encyclopedia
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
formed from two species, at least one of which is in an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
ic excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
. The lifetime of an excimer is very short, on the order of nanosecond
Nanosecond
A nanosecond is one billionth of a second . One nanosecond is to one second as one second is to 31.7 years.The word nanosecond is formed by the prefix nano and the unit second. Its symbol is ns....
s. Binding of a larger number of excited atoms form Rydberg matter
Rydberg matter
Rydberg matter is a phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and nitrogen; studies have been conducted on theoretical possibilities like...
clusters, the lifetime of which can exceed many seconds.
Formation and decay
Under the molecular orbitalMolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
formalism, a typical ground-state molecule has electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in the lowest possible energy levels. According to the Pauli principle, at most two electrons can occupy a given orbital, and if an orbital contains two electrons they must be in opposite spin states
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
. The highest occupied molecular orbital is called the HOMO and the lowest unoccupied molecular orbital is called the LUMO; the energy gap between these two states is known as the HOMO/LUMO
HOMO/LUMO
HOMO and LUMO are acronyms for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap...
gap. If the molecule absorbs light whose energy is larger than this gap, an electron in the HOMO may be excited to the LUMO. This is called the molecule's excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
.
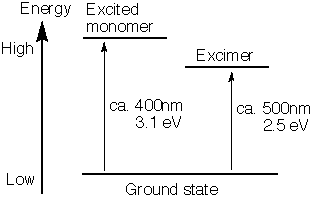
Excimers are only formed when one of the dimer components is in the excited state. When the excimer returns to the ground state, its components dissociate and often repel each other. The wavelength of an excimer's emission is longer (smaller energy) than that of the excited monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
's emission. An excimer can thus be measured by fluorescent emissions.
Because excimer formation is dependent on a bimolecular interaction, it is promoted by high monomer density. Low-density conditions produce excited monomers that decay to the ground state before they interact with an unexcited monomer to form an excimer.
Usage note
The term excimer (excited state complex) is, strictly speaking, limited to cases in which a true dimer is formed; that is, both components of the dimer are the same molecule or atom. The term exciplex refers to the heterodimeric case; however, common usage expands excimer to cover this situation.Examples and use
Heterodimeric diatomic complexes involving a noble gasNoble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
and a halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
, such as xenon chloride, are common in the construction of excimer laser
Excimer laser
An excimer laser is a form of ultraviolet laser which is commonly used in the production of microelectronic devices , eye surgery, and micromachining....
s, which are excimers' most common application. These lasers take advantage of the fact that excimer components have attractive interactions in the excited state and repulsive interactions in the ground state. The molecule pyrene
Pyrene
Pyrene is a polycyclic aromatic hydrocarbon consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is . This colourless solid is the smallest peri-fused PAH...
is another canonical example of an excimer that has found applications in biophysics to evaluate the distance between biomolecules.
In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
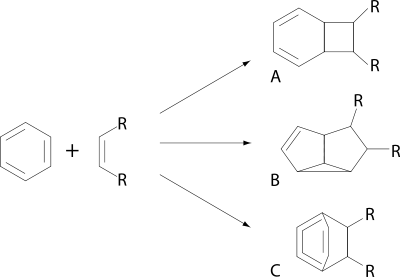
many reactions occur through an exciplex for example those of simple arene compounds with alkenes: The reactions of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and their products depicted are a [2+2]cycloaddition to the ortho product (A) ., a [2+3]cycloaddition to the meta product (B) and the [2+4]cycloaddition to the para product (C) with simple alkenes such as the isomers of 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
. In these reactions it is the arene that is excited.
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
is in favor of the ortho adduct at the expense of the meta adduct when the amount of charge transfer taking place in the exciplex increases.
See also
- Förster resonance energy transfer
- Excimer laserExcimer laserAn excimer laser is a form of ultraviolet laser which is commonly used in the production of microelectronic devices , eye surgery, and micromachining....
- Krypton fluoride laserKrypton fluoride laserA krypton fluoride laser is a particular type of excimer laser, which is sometimes called an exciplex laser. With its 248 nanometer wavelength, it is a deep ultraviolet laser which is commonly used in the production of semiconductor integrated circuits, industrial micromachining, and scientific...

