
Endergonic reaction
Encyclopedia
In chemical thermodynamics
, an endergonic reaction (also called an unfavorable reaction or a nonspontaneous reaction) is a chemical reaction
in which the standard change in free energy
is positive, and energy is absorbed. In layman's terms the total amount of energy is a loss (it takes more energy to start the reaction than what you get out of it) so the total energy is a negative net result. For an overall gain in the net result see Exergonic Reaction
.
Under constant temperature and constant pressure conditions, this means that the change in the standard Gibbs free energy
would be positive
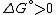
for the reaction at standard state
(ie at standard pressure (1 bar
), and standard concentrations (1 molar) of all the reagents).
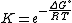
where T is the absolute temperature and R is the gas constant
. A positive value of ΔG° therefore implies
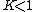
so that starting from molar stoichiometric quantities such a reaction would move backwards toward equilibrium, not forwards.
Nevertheless, endergonic reactions are quite common in nature, especially in biochemistry
and physiology
. Examples of endergonic reactions in cells include protein synthesis, and the Na+/K+ pump
which drives nerve conduction
and muscle contraction
.
(stability increasing, negative change in Free Energy) process.
A classic example of this might be the first stage of a reaction which proceeds via a transition state
. The process of getting to the top of the activation energy barrier
to the transition state is endergonic. However, the reaction can proceed because having reached the transition state, it rapidly evolves via an exergonic process to the more stable final products.
This is often how biological reactions proceed. For example, on its own the reaction
may be too endergonic to occur. However it may be possible to make it occur by coupling it to a strongly exergonic reaction – such as, very often, the decomposition of ATP
into ADP
and inorganic phosphate ions, ATP → ADP + Pi, so that

This kind of reaction, with the ATP decomposition supplying the free energy needed to make an endergonic reaction occur, is so common in cell biochemistry that ATP is often called the "universal energy currency" of all living organisms.
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
, an endergonic reaction (also called an unfavorable reaction or a nonspontaneous reaction) is a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in which the standard change in free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
is positive, and energy is absorbed. In layman's terms the total amount of energy is a loss (it takes more energy to start the reaction than what you get out of it) so the total energy is a negative net result. For an overall gain in the net result see Exergonic Reaction
Exergonic reaction
An exergonic reaction is a chemical reaction where the change in the Gibbs free energy is negative, indicating a spontaneous reaction. Symbolically, the release of Gibbs free energy, G, in an exergonic reaction is denoted as...
.
Under constant temperature and constant pressure conditions, this means that the change in the standard Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
would be positive
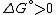
for the reaction at standard state
Standard state
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry recommends a conventional set of standard states...
(ie at standard pressure (1 bar
Bar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
), and standard concentrations (1 molar) of all the reagents).
Equilibrium constant
The equilibrium constant for the reaction is related to ΔG° by the relation: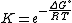
where T is the absolute temperature and R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
. A positive value of ΔG° therefore implies
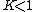
so that starting from molar stoichiometric quantities such a reaction would move backwards toward equilibrium, not forwards.
Nevertheless, endergonic reactions are quite common in nature, especially in biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
and physiology
Physiology
Physiology is the science of the function of living systems. This includes how organisms, organ systems, organs, cells, and bio-molecules carry out the chemical or physical functions that exist in a living system. The highest honor awarded in physiology is the Nobel Prize in Physiology or...
. Examples of endergonic reactions in cells include protein synthesis, and the Na+/K+ pump
Na+/K+-ATPase
Na+/K+-ATPase is an enzyme located in the plasma membrane in all animals.- Sodium-potassium pumps :Active transport is responsible for cells containing relatively high...
which drives nerve conduction
Action potential
In physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
and muscle contraction
Muscle contraction
Muscle fiber generates tension through the action of actin and myosin cross-bridge cycling. While under tension, the muscle may lengthen, shorten, or remain the same...
.
Making Endergonic reactions happen
Endergonic reactions can be achieved if they are either pulled or pushed by an exergonicExergonic
Exergonic means "releasing energy in the form of work". By thermodynamic standards, work, a form of energy, is defined as moving from the system to the surroundings...
(stability increasing, negative change in Free Energy) process.
Pull
Reagents can be pulled through an endergonic reaction, if the reaction products are cleared rapidly by a subsequent exergonic reaction. The concentration of the products of the endergonic reaction thus always remains low, so the reaction can proceed.A classic example of this might be the first stage of a reaction which proceeds via a transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
. The process of getting to the top of the activation energy barrier
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
to the transition state is endergonic. However, the reaction can proceed because having reached the transition state, it rapidly evolves via an exergonic process to the more stable final products.
Push
Endergonic reactions can be pushed by coupling them to another reaction which is strongly exergonic, through a shared intermediate.This is often how biological reactions proceed. For example, on its own the reaction

may be too endergonic to occur. However it may be possible to make it occur by coupling it to a strongly exergonic reaction – such as, very often, the decomposition of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
into ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
and inorganic phosphate ions, ATP → ADP + Pi, so that


This kind of reaction, with the ATP decomposition supplying the free energy needed to make an endergonic reaction occur, is so common in cell biochemistry that ATP is often called the "universal energy currency" of all living organisms.
See also
- EndergonicEndergonicEndergonic means "absorbing energy in the form of work." Endergonic reactions are not spontaneous...
- ExergonicExergonicExergonic means "releasing energy in the form of work". By thermodynamic standards, work, a form of energy, is defined as moving from the system to the surroundings...
- Exergonic reactionExergonic reactionAn exergonic reaction is a chemical reaction where the change in the Gibbs free energy is negative, indicating a spontaneous reaction. Symbolically, the release of Gibbs free energy, G, in an exergonic reaction is denoted as...
- ExothermicExothermicIn thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
- EndothermicEndothermicIn thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
- Exothermic reactionExothermic reactionAn exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
- Endothermic reaction
- EndothermWarm-bloodedThe term warm-blooded is a colloquial term to describe animal species which have a relatively higher blood temperature, and maintain thermal homeostasis primarily through internal metabolic processes...
- ExothermWarm-bloodedThe term warm-blooded is a colloquial term to describe animal species which have a relatively higher blood temperature, and maintain thermal homeostasis primarily through internal metabolic processes...

