
Electron rest mass
Encyclopedia
| Values of me | Units |
|---|---|
| 9.109 382 15(45) | kg Kilogram The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water... |
| 5.485 799 0943(23) | u Atomic mass unit The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of... |
| 8.187 104 38(41) | J/c2 Joule The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second... |
| 0.510 998 910(13) | MeV/c2 Electronvolt In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt... |
| Values of the energy of me | Units |
| 8.187 104 38(41) | J Joule The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second... |
| 0.510 998 910(13) | MeV Electronvolt In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt... |
The electron rest mass (symbol: me) is the mass
Mass in special relativity
Mass in special relativity incorporates the general understandings from the concept of mass-energy equivalence. Added to this concept is an additional complication resulting from the fact that "mass" is defined in two different ways in special relativity: one way defines mass as an invariant...
of a stationary electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
. It is one of the fundamental constants
Physical constant
A physical constant is a physical quantity that is generally believed to be both universal in nature and constant in time. It can be contrasted with a mathematical constant, which is a fixed numerical value but does not directly involve any physical measurement.There are many physical constants in...
of physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, and is also very important in chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
because of its relation to the Avogadro constant. It has a value of about 9.11 kilogram
Kilogram
The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water...
s or about 5.486 atomic mass unit
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
s, equivalent to an energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
of about 8.19 joule
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s or about 0.511 megaelectronvolts
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
.
Terminology
The term "rest mass" comes from the need to take account of the effects of special relativitySpecial relativity
Special relativity is the physical theory of measurement in an inertial frame of reference proposed in 1905 by Albert Einstein in the paper "On the Electrodynamics of Moving Bodies".It generalizes Galileo's...
on the apparent (or "observed") mass of an electron. It is impossible to "weigh" a stationary electron, and so all practical measurements must be carried out on moving electrons. However special relativity shows that the mass of an object appears to increase as its speed v (relative to the observer) approaches the speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
c. The 2006 CODATA recommended value for the electron rest mass (in atomic units) has a measurement uncertainty
Measurement uncertainty
In metrology, measurement uncertainty is a non-negative parameter characterizing the dispersion of the values attributed to a measured quantity. The uncertainty has a probabilistic basis and reflects incomplete knowledge of the quantity. All measurements are subject to uncertainty and a measured...
of 4.2: this is equivalent to relativistic correction to the observed mass of a body travelling at 3c, or about 9000 m/s. Although this is a high speed in normal experience, it is very low in terms of subatomic particles: for an electron, it corresponds to a kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of 2.15 eV, or the energy gained by an electron when it is accelerated through a potential difference of just 0.215 mV
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
.
Electron relative atomic mass
In metrologyMetrology
Metrology is the science of measurement. Metrology includes all theoretical and practical aspects of measurement. The word comes from Greek μέτρον , "measure" + "λόγος" , amongst others meaning "speech, oration, discourse, quote, study, calculation, reason"...
, the electron rest mass can refer to two separate and independent quantities. The first is the rest mass of an electron in SI units (or any other macroscopic system of units). The second is the rest mass of the electron on the scale used to measure relative atomic masses, where the mass of an atom of carbon 12 has a value of exactly 12. CODATA refers to this second quantity as the electron relative atomic mass, with the symbol Ar(e), regardless that the electron is not an atom. The US National Institute of Standards and Technology
National Institute of Standards and Technology
The National Institute of Standards and Technology , known between 1901 and 1988 as the National Bureau of Standards , is a measurement standards laboratory, otherwise known as a National Metrological Institute , which is a non-regulatory agency of the United States Department of Commerce...
(NIST) refers to the quantity as the electron mass in u (atomic mass units), but this term involves a circular definition as discussed below.
The distinction is important because the two quantities are determined by completely different methods, so the "electron mass in u" is not calculated from the electron mass in kilograms, or vice versa. The ratio of the two quantities, multiplied by a constant factor, gives the Avogadro constant NA:
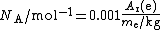
The factor of 0.001 is the molar mass constant
Molar mass constant
The molar mass constant, symbol Mu, is a physical constant which relates atomic weight and molar mass. Its value is defined to be 1 g/mol in SI units....
, Mu, which is defined as 0.001 kg/mol in SI units.
Determination
The electron rest mass in kilograms is calculated from the definition of the Rydberg constantRydberg constant
The Rydberg constant, symbol R∞, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to atomic spectra in the science of spectroscopy. Rydberg initially determined its value empirically from spectroscopy, but Niels Bohr later showed that its value could be calculated...
R∞:
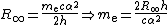
where α is the fine structure constant and h is the Planck constant
Planck constant
The Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
. The relative uncertainty, 5 in the 2006 CODATA recommended value, is due entirely to the uncertainty in the value of the Planck constant.
The electron relative atomic mass can be measured directly in a Penning trap
Penning trap
Penning traps are devices for the storage of charged particles using a homogeneous static magnetic field and a spatially inhomogeneous static electric field. This kind of trap is particularly well suited to precision measurements of properties of ions and stable subatomic particles which have...
. It can also be inferred from the spectra of antiprotonic helium
Antiprotonic helium
Antiprotonic helium is a three-body atom composed of an antiproton and an electron orbiting around a helium nucleus. It is thus made partly of matter, and partly of antimatter. The atom is electrically neutral, since both electrons and antiprotons have a charge of -1, whereas helium nuclei have a...
atoms (helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
atoms where one of the electrons has been replaced by an antiproton
Antiproton
The antiproton is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived since any collision with a proton will cause both particles to be annihilated in a burst of energy....
) or from measurements of the electron g-factor
G-factor
A g-factor is a dimensionless quantity which characterizes the magnetic moment and gyromagnetic ratio of a particle or nucleus...
in the hydrogenic ions 12C5+ or 16O7+. The 2006 CODATA recommended value has a relative uncertainty of 4.2.
The electron relative atomic mass is an adjusted parameter in the CODATA set of fundamental physical constants, while the electron rest mass in kilograms is calculated from the values of the Planck constant, the fine structure constant and the Rydberg constant. The correlation between the two values is negligible (r = 0.0003).
Relationship to other physical constants
As mentioned above, the electron mass is used to calculate the Avogadro constant NA: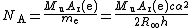
Hence it is also related to the atomic mass constant
Atomic mass constant
In physics and chemistry, the atomic mass constant, mu, is one twelfth of the mass of an unbound atom of carbon-12 at rest and in its ground state. It serves to define the atomic mass unit and is, by definition, equal to 1 u...
mu:
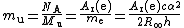
Note that mu is defined in terms of Ar(e), and not the other way round, and so the name "electron mass in atomic mass units" for Ar(e) involves a circular definition (at least in terms of practical measurements).
The electron relative atomic mass also enters into the calculation of all other relative atomic masses. By convention, relative atomic masses are quoted for neutral atoms, but the actual measurements are made on positive ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s, either in a mass spectrometer or a Penning trap
Penning trap
Penning traps are devices for the storage of charged particles using a homogeneous static magnetic field and a spatially inhomogeneous static electric field. This kind of trap is particularly well suited to precision measurements of properties of ions and stable subatomic particles which have...
. Hence the mass of the electrons must be added back on to the measured values before tabulation. A correction must also be made for the mass equivalent of the binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
Eb. Taking the simplest case of complete ionization of all electrons, for a nuclide X of atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
Z,
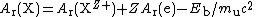
As relative atomic masses are measured as ratios of masses, the corrections must be applied to both ions: fortunately, the uncertainties in the corrections are negligible, as illustrated below for hydrogen 1 and oxygen 16.
| 1H | 16O | |
|---|---|---|
| relative atomic mass of the XZ+ ion | 1.007 276 466 77(10) | 15.990 528 174 45(18) |
| relative atomic mass of the Z electrons | 0.000 548 579 909 43(23) | 0.004 388 639 2754(18) |
| correction for the binding energy | −0.000 000 014 5985 | −0.000 002 194 1559 |
| relative atomic mass of the neutral atom | 1.007 825 032 07(10) | 15.994 914 619 57(18) |
The principle can be shown by the determination of the electron relative atomic mass by Farnham et al. at the University of Washington (1995). It involves the measurement of the frequencies of the cyclotron radiation
Cyclotron radiation
Cyclotron radiation is electromagnetic radiation emitted by moving charged particles deflected by a magnetic field. The Lorentz force on the particles acts perpendicular to both the magnetic field lines and the particles' motion through them, creating an acceleration of charged particles that...
emitted by electrons and by 12C6+ ions in a Penning trap. The ratio of the two frequencies is equal to six times the inverse ratio of the masses of the two particles (the heavier the particle, the lower the frequency of the cyclotron radiation; the higher the charge on the particle, the higher the frequency):
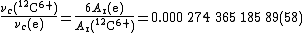
As the relative atomic mass of 12C6+ ions is very nearly 12, the ratio of frequencies can be used to calculate a first approximation to Ar(e), . This approximate value is then used to calculate a first approximation to Ar(12C6+), knowing that Eb(12C)/muc2 (from the sum of the six ionization energies of carbon) is : Ar(12C6+) ≈ . This value is then used to calculate a new approximation to Ar(e), and the process repeated until the values no longer vary (given the relative uncertainty of the measurement, 2.1): this happens by the fourth cycle of iterations for these results, giving Ar(e) = for these data.

