
Electron bubble
Encyclopedia
An electron bubble is the empty space created around a free electron
in a cryogenic gas or liquid, such as neon
or helium
. They are typically very small, about 2 nm in diameter at atmospheric pressure.
ses move about freely, limited only by collisions with the weakly interacting atoms. Their mobility
, which depends on the gas density and temperature, is well described by classical kinetic theory
. As the temperature is lowered the electron mobility decreases, since the helium atoms slow down at lower temperature and do not interact with the electron as often[1].
Below a critical temperature, the mobility of the electrons drops quickly to a value much below what is expected classically. This discrepancy led to the development of the electron bubble theory[2]. At low temperatures, electrons injected into liquid helium
do not move freely as one might expect, but rather form small vacuum bubbles around themselves.
s between the gas and liquid phase
of helium. The negative electron polarizes the helium at the surface, leading to an image charge which binds it to the surface. The electron is forbidden from entering the liquid for the same reason hydrogen
atoms are stable: quantum mechanics
. The electron and image charge form a bound state
, just as an electron and proton
do in a hydrogen atom, with a minimum average separation. In this case, the minimum energy is about 1 eV
(a moderate amount of energy on an atomic scale)[3].
When an electron is forced into liquid helium rather than floating on its surface, it forms a bubble rather than entering the liquid. The size of this bubble is determined by three main factors (ignoring small corrections): the confinement term, the surface tension
term, and the pressure-volume term. The confinement term is purely quantum mechanical, since whenever an electron is tightly confined, its kinetic energy
goes up. The surface tension term represents the surface energy
of the liquid helium; this is exactly like water and all other liquids. The pressure-volume term is the amount of energy needed to push the helium out of the bubble[4].
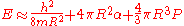
Here E is the energy of the bubble, h is Planck's constant, m is the electron mass, R is the bubble radius, α is the surface energy, and P is the ambient pressure.
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in a cryogenic gas or liquid, such as neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
or helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. They are typically very small, about 2 nm in diameter at atmospheric pressure.
Electron bubbles in helium
At room temperature, electrons in noble gasNoble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
ses move about freely, limited only by collisions with the weakly interacting atoms. Their mobility
Electron mobility
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor, when pulled by an electric field. In semiconductors, there is an analogous quantity for holes, called hole mobility...
, which depends on the gas density and temperature, is well described by classical kinetic theory
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
. As the temperature is lowered the electron mobility decreases, since the helium atoms slow down at lower temperature and do not interact with the electron as often[1].
Below a critical temperature, the mobility of the electrons drops quickly to a value much below what is expected classically. This discrepancy led to the development of the electron bubble theory[2]. At low temperatures, electrons injected into liquid helium
Liquid helium
Helium exists in liquid form only at extremely low temperatures. The boiling point and critical point depend on the isotope of the helium; see the table below for values. The density of liquid helium-4 at its boiling point and 1 atmosphere is approximately 0.125 g/mL Helium-4 was first liquefied...
do not move freely as one might expect, but rather form small vacuum bubbles around themselves.
Electron repulsion from the surface of helium
Electrons are attracted to liquid helium due to the difference in dielectric constantDielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
s between the gas and liquid phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
of helium. The negative electron polarizes the helium at the surface, leading to an image charge which binds it to the surface. The electron is forbidden from entering the liquid for the same reason hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atoms are stable: quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
. The electron and image charge form a bound state
Bound state
In physics, a bound state describes a system where a particle is subject to a potential such that the particle has a tendency to remain localised in one or more regions of space...
, just as an electron and proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
do in a hydrogen atom, with a minimum average separation. In this case, the minimum energy is about 1 eV
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
(a moderate amount of energy on an atomic scale)[3].
When an electron is forced into liquid helium rather than floating on its surface, it forms a bubble rather than entering the liquid. The size of this bubble is determined by three main factors (ignoring small corrections): the confinement term, the surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
term, and the pressure-volume term. The confinement term is purely quantum mechanical, since whenever an electron is tightly confined, its kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
goes up. The surface tension term represents the surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
of the liquid helium; this is exactly like water and all other liquids. The pressure-volume term is the amount of energy needed to push the helium out of the bubble[4].
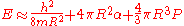
Here E is the energy of the bubble, h is Planck's constant, m is the electron mass, R is the bubble radius, α is the surface energy, and P is the ambient pressure.
The 2S electron bubble
A theoretical prediction has been made based on the analysis of the equation above [5], that the 2S electron bubble exhibits a startling morphological instability under a wide range of ambient pressures. While its wave function is spherical, the stable shape of the bubble is nonspherical.Footnotes
- 1.
- 2.
- 3.
- 4.
- 5.

