Electric glow discharge
Encyclopedia
An electric glow discharge is a plasma
formed by the passage of current at 100 V
to several kV through a gas, often argon
or another noble gas
. It is found in products such as neon lamp
s and plasma-screen televisions
, and is used in plasma physics and analytical chemistry
.
 This system was first made by William Crookes
This system was first made by William Crookes
.
The simplest type of glow discharge is a direct-current glow discharge. In its simplest form, it consists of two electrodes in a cell held at low pressure (0.1–10 torr
; about 1/10000th to 1/100th of atmospheric pressure). The cell is typically filled with neon, but other gases can also be used. An electric potential
of several hundred volts is applied between the two electrodes. A small fraction of the population of atoms within the cell is initially ionized through random processes (thermal collisions between atoms or with alpha particle
s, for example). The ions (which are positively charged) are driven towards the cathode
by the electric potential, and the electrons are driven towards the anode
by the same potential. The initial population of ions and electrons collides with other atoms, ionizing them. As long as the potential is maintained, a population of ions and electrons remains.
 Some of the ions' kinetic energy is transferred to the cathode. This happens partially through the ions striking the cathode directly. The primary mechanism, however, is less direct. Ions strike the more numerous neutral gas atoms, transferring a portion of their energy to them. These neutral atoms then strike the cathode. Whichever species (ions or atoms) strike the cathode, collisions within the cathode redistribute this energy until a portion of the cathode is ejected, typically in the form of free atoms. This process is known as sputtering
Some of the ions' kinetic energy is transferred to the cathode. This happens partially through the ions striking the cathode directly. The primary mechanism, however, is less direct. Ions strike the more numerous neutral gas atoms, transferring a portion of their energy to them. These neutral atoms then strike the cathode. Whichever species (ions or atoms) strike the cathode, collisions within the cathode redistribute this energy until a portion of the cathode is ejected, typically in the form of free atoms. This process is known as sputtering
. Once free of the cathode, atoms move into the bulk of the glow discharge through drift and due to the energy they gained from sputtering. The atoms can then be excited by collisions with ions, electrons, or other atoms that have been previously excited by collisions. Once excited, atoms will lose their energy fairly quickly. Of the various ways that this energy can be lost, the most important is radiatively, meaning that a photon is released to carry the energy away. In optical atomic spectroscopy
, the wavelength of this photon can be used to determine the identity of the atom (that is, which chemical element
it is) and the number of photons is directly proportional to the concentration of that element in the sample. Some collisions (those of high enough energy) will cause ionization. In atomic mass spectrometry
, these ions are detected. Their mass identifies the type of atoms and their quantity reveals the amount of that element in the sample.
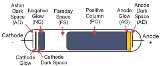
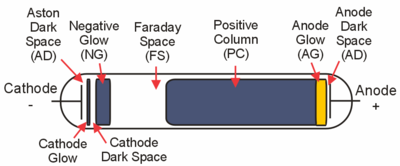
The figure above shows the main regions that may be present in a glow discharge. Regions described as "glows" emit significant light; regions labeled as "dark spaces" do not. As the discharge becomes more extended (i.e., stretched horizontally in the geometry of the figure), the positive column may become striated. That is, alternating dark and bright regions may form. Compressing the discharge horizontally will result in fewer regions. The positive column will be compressed while the negative glow will remain the same size, and, with small enough gaps, the positive column will disappear altogether. In an analytical glow discharge, the discharge is primarily a negative glow with dark region above and below it.
Below the ionization voltage or breakdown voltage
there is no glow, but as the voltage increases to the ionization point the Townsend discharge
happens just as glow discharge becomes visible; this is the start of the normal glow range. As the voltage is increased above the normal glow range, abnormal glow begins. If the voltage is increased to the point the cathode glow covers the entire cathode arc discharge
begins.
Both bulk and depth analysis of solids may be performed with glow discharge. Bulk analysis assumes that the sample is fairly homogeneous and averages the emission or mass spectrometric signal over time. Depth analysis relies on the fact that the depth increases as time goes by. Tracking the signal in time, therefore, is the same as tracking the elemental composition in depth. Depth analysis requires greater control over operational parameters. For example, conditions (current, potential, pressure) need to be adjusted so that the crater produced by sputtering is flat bottom (that is, so that the depth analyzed over the crater area is uniform). In bulk measurement, a rough or rounded crater bottom would not adversely impact analysis. Under the best conditions, depth resolution in the single nanometer range has been achieved (in fact, within-molecule resolution has been demonstrated).
The chemistry of ions and neutrals in vacuum is called gas phase ion chemistry
and is part of the analytical study that includes Electric glow discharge.
Glow discharges may also be operated in radio-frequency. The use of this frequency will establish a negative DC-bias voltage on the sample surface. The DC-bias is the result of an alternating current waveform that is centered about negative potential; as such it more or less represent the average potential residing on the sample surface. Radio-frequency has ability to appear to flow through insulators (non-conductive materials).
Both radio-frequency and direct-current glow discharges can be operated in pulsed mode, where the potential is turned on and off. This allows higher instantaneous powers to be applied without excessively heating the cathode. These higher instantaneous powers produce higher instantaneous signals, aiding detection. Combining time-resolved detection with pulsed powering results in additional benefits. In atomic emission, analyte atoms emit during different portions of the pulse than background atoms, allowing the two to be discriminated. Analogously, in mass spectrometry, sample and background ions are created at different times.
glow discharge, hollow cathode discharge, spray discharge.
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
formed by the passage of current at 100 V
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
to several kV through a gas, often argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
or another noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
. It is found in products such as neon lamp
Neon lamp
A neon lamp is a miniature gas discharge lamp that typically contains neon gas at a low pressure in a glass capsule. Only a thin region adjacent to the electrodes glows in these lamps, which distinguishes them from the much longer and brighter neon tubes used for signage...
s and plasma-screen televisions
Plasma display
A plasma display panel is a type of flat panel display common to large TV displays or larger. They are called "plasma" displays because the technology utilizes small cells containing electrically charged ionized gases, or what are in essence chambers more commonly known as fluorescent...
, and is used in plasma physics and analytical chemistry
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
.
Basic operating mechanism

William Crookes
Sir William Crookes, OM, FRS was a British chemist and physicist who attended the Royal College of Chemistry, London, and worked on spectroscopy...
.
The simplest type of glow discharge is a direct-current glow discharge. In its simplest form, it consists of two electrodes in a cell held at low pressure (0.1–10 torr
Torr
The torr is a non-SI unit of pressure with the ratio of 760 to 1 standard atmosphere, chosen to be roughly equal to the fluid pressure exerted by a millimetre of mercury, i.e., a pressure of 1 torr is approximately equal to 1 mmHg...
; about 1/10000th to 1/100th of atmospheric pressure). The cell is typically filled with neon, but other gases can also be used. An electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
of several hundred volts is applied between the two electrodes. A small fraction of the population of atoms within the cell is initially ionized through random processes (thermal collisions between atoms or with alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s, for example). The ions (which are positively charged) are driven towards the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
by the electric potential, and the electrons are driven towards the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
by the same potential. The initial population of ions and electrons collides with other atoms, ionizing them. As long as the potential is maintained, a population of ions and electrons remains.

Sputtering
Sputtering is a process whereby atoms are ejected from a solid target material due to bombardment of the target by energetic particles. It is commonly used for thin-film deposition, etching and analytical techniques .-Physics of sputtering:...
. Once free of the cathode, atoms move into the bulk of the glow discharge through drift and due to the energy they gained from sputtering. The atoms can then be excited by collisions with ions, electrons, or other atoms that have been previously excited by collisions. Once excited, atoms will lose their energy fairly quickly. Of the various ways that this energy can be lost, the most important is radiatively, meaning that a photon is released to carry the energy away. In optical atomic spectroscopy
Atomic spectroscopy
Atomic spectroscopy is the determination of elemental composition by its electromagnetic or mass spectrum. Atomic spectroscopy is closely related to other forms of spectroscopy. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is...
, the wavelength of this photon can be used to determine the identity of the atom (that is, which chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
it is) and the number of photons is directly proportional to the concentration of that element in the sample. Some collisions (those of high enough energy) will cause ionization. In atomic mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
, these ions are detected. Their mass identifies the type of atoms and their quantity reveals the amount of that element in the sample.
The figure above shows the main regions that may be present in a glow discharge. Regions described as "glows" emit significant light; regions labeled as "dark spaces" do not. As the discharge becomes more extended (i.e., stretched horizontally in the geometry of the figure), the positive column may become striated. That is, alternating dark and bright regions may form. Compressing the discharge horizontally will result in fewer regions. The positive column will be compressed while the negative glow will remain the same size, and, with small enough gaps, the positive column will disappear altogether. In an analytical glow discharge, the discharge is primarily a negative glow with dark region above and below it.
Below the ionization voltage or breakdown voltage
Breakdown voltage
The breakdown voltage of an insulator is the minimum voltage that causes a portion of an insulator to become electrically conductive.The breakdown voltage of a diode is the minimum reverse voltage to make the diode conduct in reverse...
there is no glow, but as the voltage increases to the ionization point the Townsend discharge
Townsend discharge
The Townsend discharge is a gas ionization process where an initially very small amount of free electrons, accelerated by a sufficiently strong electric field, give rise to electrical conduction through a gas by avalanche multiplication: when the number of free charges drops or the electric field...
happens just as glow discharge becomes visible; this is the start of the normal glow range. As the voltage is increased above the normal glow range, abnormal glow begins. If the voltage is increased to the point the cathode glow covers the entire cathode arc discharge
Electric arc
An electric arc is an electrical breakdown of a gas which produces an ongoing plasma discharge, resulting from a current flowing through normally nonconductive media such as air. A synonym is arc discharge. An arc discharge is characterized by a lower voltage than a glow discharge, and relies on...
begins.
Use in analytical chemistry
Glow discharges can be used to analyze the elemental, and sometimes molecular, composition of solids, liquids, and gases, but elemental analysis of solids is by far the most common. In this arrangement, the sample is used as the cathode. As mentioned earlier, gas ions and atoms striking the sample surface knock atoms off of it (a process known as sputtering). The sputtered atoms, now in the gas phase, can be detected by atomic absorption, but this is a comparatively rare strategy. Instead, atomic emission and mass spectrometry are usually used. Collisions between the gas-phase sample atoms and the plasma gas pass energy to the sample atoms. This energy can excite the atoms, after which they can lose their energy through atomic emission. By observing the wavelength of the emitted light, the atom's identity can be determined. By observing the intensity of the emission, the concentration of atoms of that type can be determined. Energy gained through collisions can also ionize the sample atoms. The ions can then be detected by mass spectrometry. In this case, it is the mass of the ions that identified the element and the number of ions that reflects the concentrationin. This method is referred to as glow discharge mass spectrometry (GDMS) and it has detection limits down to the sub-ppb range for most elements that are nearly matrix-independent.Both bulk and depth analysis of solids may be performed with glow discharge. Bulk analysis assumes that the sample is fairly homogeneous and averages the emission or mass spectrometric signal over time. Depth analysis relies on the fact that the depth increases as time goes by. Tracking the signal in time, therefore, is the same as tracking the elemental composition in depth. Depth analysis requires greater control over operational parameters. For example, conditions (current, potential, pressure) need to be adjusted so that the crater produced by sputtering is flat bottom (that is, so that the depth analyzed over the crater area is uniform). In bulk measurement, a rough or rounded crater bottom would not adversely impact analysis. Under the best conditions, depth resolution in the single nanometer range has been achieved (in fact, within-molecule resolution has been demonstrated).
The chemistry of ions and neutrals in vacuum is called gas phase ion chemistry
Gas phase ion chemistry
Gas phase ion chemistry is a field of science encompassed within both chemistry and physics. It is the science that studies ions and molecules in the gas phase, most often enabled by some form of mass spectrometry. By far the most important applications for this science is in studying the...
and is part of the analytical study that includes Electric glow discharge.
Powering modes
In analytical chemistry, glow discharges are most often operated in direct-current mode. For this mode, the cathode (which is the sample in solids analysis) must be conductive. The potential, pressure, and current are interrelated. Only two can be directly controlled at once, while the third must be allowed to vary. The pressure is most typically held constant, but other schemes may be used. The pressure and current may be held constant, while potential is allowed to vary. The pressure and voltage may be held constant while the current is allowed to vary. The power (product of voltage and current) may be held constant while the pressure is allowed to vary.Glow discharges may also be operated in radio-frequency. The use of this frequency will establish a negative DC-bias voltage on the sample surface. The DC-bias is the result of an alternating current waveform that is centered about negative potential; as such it more or less represent the average potential residing on the sample surface. Radio-frequency has ability to appear to flow through insulators (non-conductive materials).
Both radio-frequency and direct-current glow discharges can be operated in pulsed mode, where the potential is turned on and off. This allows higher instantaneous powers to be applied without excessively heating the cathode. These higher instantaneous powers produce higher instantaneous signals, aiding detection. Combining time-resolved detection with pulsed powering results in additional benefits. In atomic emission, analyte atoms emit during different portions of the pulse than background atoms, allowing the two to be discriminated. Analogously, in mass spectrometry, sample and background ions are created at different times.
Types
There are various types of glow discharge examples include: high pressureglow discharge, hollow cathode discharge, spray discharge.
See also
- Electric arcElectric arcAn electric arc is an electrical breakdown of a gas which produces an ongoing plasma discharge, resulting from a current flowing through normally nonconductive media such as air. A synonym is arc discharge. An arc discharge is characterized by a lower voltage than a glow discharge, and relies on...
discharge - Vacuum arcVacuum arcA vacuum arc can arise when the surfaces of metal electrodes in contact with a good vacuum begin to emit electrons either through heating or via an electric field that is sufficient to cause field electron emission...
- X-ray tubeX-ray tubeAn X-ray tube is a vacuum tube that produces X-rays. They are used in X-ray machines. X-rays are part of the electromagnetic spectrum, an ionizing radiation with wavelengths shorter than ultraviolet light...
- Fluorescent lampFluorescent lampA fluorescent lamp or fluorescent tube is a gas-discharge lamp that uses electricity to excite mercury vapor. The excited mercury atoms produce short-wave ultraviolet light that then causes a phosphor to fluoresce, producing visible light. A fluorescent lamp converts electrical power into useful...
, neon lampNeon lampA neon lamp is a miniature gas discharge lamp that typically contains neon gas at a low pressure in a glass capsule. Only a thin region adjacent to the electrodes glows in these lamps, which distinguishes them from the much longer and brighter neon tubes used for signage...
, and plasma lampPlasma lampPlasma globes, or plasma lamps , are novelty items that were most popular in the 1980s...

