Doebner-Miller reaction
Encyclopedia
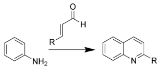
The Doebner-Miller reaction is the organic reaction
of an aniline
with α,β-unsaturated carbonyl compounds to form quinoline
s.
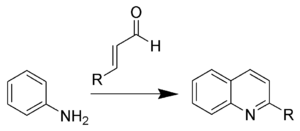 This reaction is also known as the Skraup-Doebner-Von Miller quinoline synthesis, and is named after the Czech chemist Zdenko Hans Skraup
This reaction is also known as the Skraup-Doebner-Von Miller quinoline synthesis, and is named after the Czech chemist Zdenko Hans Skraup
(1850-1910), and the Germans Oscar Döbner (Doebner) (1850-1907) and Wilhelm von Miller (1848-1899). When the α,β-unsaturated carbonyl
compound is prepared in situ
from two carbonyl compounds (via an Aldol condensation
), the reaction is known as the Beyer method for quinolines.
The reaction is catalyzed by lewis acid
s such as tin tetrachloride and scandium(III) triflate
and bronsted acids such as p-toluenesulfonic acid
, perchloric acid
, amberlite and iodine
.
for this reaction and the related Skraup synthesis is a matter of debate. A 2006 study proposes a fragmentation-recombination mechanism based on carbon isotope
scrambling experiments. In this study 4-isopropylaniline 1 is reacted with a mixture (50:50)of ordinary pulegone
and the 14C-enriched isomer 2 and the reaction mechanism is outlined in scheme 2 with the labeled carbon identified with a red dot. The first step is a nucleophilic conjugate addition
of the amine
with the enol
to the amine ketone 3 in a reversible reaction
. This intermediate then fragmentates to the imine
4a and the saturated cyclohexanone
4b in a non-reversible reaction and both fragments recombine in a condensation reaction
to the conjugated
imine 5. In the next step 5 reacts with a second aniline molecule in a nucleophilic conjugate addition to imine 6 and subsequent electrophilic addition
and proton transfer to leads to 7. elimination
of one aniline molecule through 8 and rearomatization leads to final product 9. Because α-amino protons are not available in this model compound the reaction is not taken to the fully fledged quinoline.
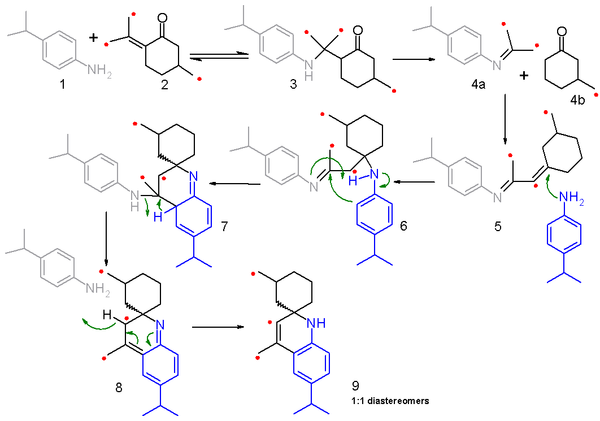 The fragmentation to 4a and 4b is key to this mechanism because it explains the isotope scrambling results. In the reaction only half the pulegone
The fragmentation to 4a and 4b is key to this mechanism because it explains the isotope scrambling results. In the reaction only half the pulegone
reactant (2) is labeled and on recombining a labeled imine fragment can react with another labeled ketone fragment or an unlabeled fragment and likewise a labeled ketone fragment can react with a labeled or unlabeled imine fragment. The resulting product distribution is confirmed by mass spectrometry
of the final product 9.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
of an aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
with α,β-unsaturated carbonyl compounds to form quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
s.

Zdenko Hans Skraup
Zdenko Hans Skraup was a Czech Austrian chemist who discovered the Skraup reaction, the first quinoline synthesis.-Life:...
(1850-1910), and the Germans Oscar Döbner (Doebner) (1850-1907) and Wilhelm von Miller (1848-1899). When the α,β-unsaturated carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compound is prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
from two carbonyl compounds (via an Aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
), the reaction is known as the Beyer method for quinolines.
The reaction is catalyzed by lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s such as tin tetrachloride and scandium(III) triflate
Scandium(III) triflate
Scandium trifluoromethanesulfonate, commonly called scandium triflate, is a chemical compound with formula Sc3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3− anions....
and bronsted acids such as p-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
, perchloric acid
Perchloric acid
Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,...
, amberlite and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for this reaction and the related Skraup synthesis is a matter of debate. A 2006 study proposes a fragmentation-recombination mechanism based on carbon isotope
Carbon-14
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues , to date archaeological, geological, and hydrogeological...
scrambling experiments. In this study 4-isopropylaniline 1 is reacted with a mixture (50:50)of ordinary pulegone
Pulegone
Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria , Mentha piperita, and pennyroyal. It is classified as a monoterpene....
and the 14C-enriched isomer 2 and the reaction mechanism is outlined in scheme 2 with the labeled carbon identified with a red dot. The first step is a nucleophilic conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
of the amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
with the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
to the amine ketone 3 in a reversible reaction
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
. This intermediate then fragmentates to the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
4a and the saturated cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
4b in a non-reversible reaction and both fragments recombine in a condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
to the conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
imine 5. In the next step 5 reacts with a second aniline molecule in a nucleophilic conjugate addition to imine 6 and subsequent electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
and proton transfer to leads to 7. elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of one aniline molecule through 8 and rearomatization leads to final product 9. Because α-amino protons are not available in this model compound the reaction is not taken to the fully fledged quinoline.

Pulegone
Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria , Mentha piperita, and pennyroyal. It is classified as a monoterpene....
reactant (2) is labeled and on recombining a labeled imine fragment can react with another labeled ketone fragment or an unlabeled fragment and likewise a labeled ketone fragment can react with a labeled or unlabeled imine fragment. The resulting product distribution is confirmed by mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
of the final product 9.
See also
- Combes quinoline synthesisCombes quinoline synthesisThe Combes quinoline synthesis is a chemical reaction involving the condensation of unsubstituted anilines with β-diketones to form substituted quinolines after an acid-catalyzed ring closure of an intermediate Schiff base .-See also:*Conrad-Limpach reaction*Doebner reaction*Doebner-Miller...
- Doebner reactionDoebner reactionThe Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids....
- Gould-Jacobs reactionGould-Jacobs reactionThe Gould-Jacobs reaction is an organic synthesis for the preparation of quinolines. In this reaction aniline or an aniline derivative first reacts with malonic acid derivative ethyl ethoxymethylenemalonate with substitution of the ethoxy group by nitrogen. A benzannulation takes place by...
- Knorr quinoline synthesisKnorr quinoline synthesisThe Knorr quinoline synthesis is an intramolecular organic reaction converting a β-ketoanilide to a 2-hydroxyquinoline using sulfuric acid. This reaction was first described by Ludwig Knorr in 1886...
- Riehm quinoline synthesis
- Skraup synthesis

