Deuterium arc lamp
Encyclopedia
A deuterium arc lamp is a low-pressure gas-discharge light source often used in spectroscopy
when a continuous spectrum
in the ultraviolet
region is needed.
 A deuterium lamp uses a tungsten
A deuterium lamp uses a tungsten
filament and anode placed on opposite sides of a nickel
box structure designed to produce the best output spectrum. Unlike an incandescent bulb, the filament is not the source of light in deuterium lamps. Instead an arc is created from the filament to the anode, a similar process to arc lamps. Because the filament must be very hot before it can operate, it is heated for approximately twenty seconds before use. Because the discharge process produces its own heat, the heater is turned down after discharge begins. Although firing voltages are 300 to 500 volts, once the arc is created voltages drop to around 100 to 200 volts.
The arc created excites the molecular deuterium contained within the bulb to a higher energy state. The deuterium then emits light as it transitions back to its initial state. This continuous cycle is the origin of the continuous ultraviolet radiation. This process is not the same as the process of decay of atomic excited states
(atomic emission), where electrons are excited and then emit radiation. Instead from the molecular emission process, where radiative decay of excited states, in this case of molecular deuterium (D2), causes the effect.
Because the lamp operates at high temperatures, normal glass housings cannot be used for a casing (which would also block UV radiation). Instead, a fused quartz
, UV glass, or magnesium fluoride
envelope is used depending on the specific function of the lamp.
The typical lifetime of a deuterium lamp is approximately 2000 hours (Most manufacturers guarantee 2000 hours, but newer lamps are consistently performing well out to 5000 hours and more).
s. Touching the bulb directly even when cool can smudge the casing and therefore reduce output intensity.
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
when a continuous spectrum
Continuous spectrum
The spectrum of a linear operator is commonly divided into three parts: point spectrum, continuous spectrum, and residual spectrum.If H is a topological vector space and A:H \to H is a linear map, the spectrum of A is the set of complex numbers \lambda such that A - \lambda I : H \to H is not...
in the ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
region is needed.
Principle of operation

Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
filament and anode placed on opposite sides of a nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
box structure designed to produce the best output spectrum. Unlike an incandescent bulb, the filament is not the source of light in deuterium lamps. Instead an arc is created from the filament to the anode, a similar process to arc lamps. Because the filament must be very hot before it can operate, it is heated for approximately twenty seconds before use. Because the discharge process produces its own heat, the heater is turned down after discharge begins. Although firing voltages are 300 to 500 volts, once the arc is created voltages drop to around 100 to 200 volts.
The arc created excites the molecular deuterium contained within the bulb to a higher energy state. The deuterium then emits light as it transitions back to its initial state. This continuous cycle is the origin of the continuous ultraviolet radiation. This process is not the same as the process of decay of atomic excited states
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
(atomic emission), where electrons are excited and then emit radiation. Instead from the molecular emission process, where radiative decay of excited states, in this case of molecular deuterium (D2), causes the effect.
Because the lamp operates at high temperatures, normal glass housings cannot be used for a casing (which would also block UV radiation). Instead, a fused quartz
Fused quartz
Fused quartz and fused silica are types of glass containing primarily silica in amorphous form. They are manufactured using several different processes...
, UV glass, or magnesium fluoride
Magnesium fluoride
Magnesium fluoride is an inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics.-Production and structure:...
envelope is used depending on the specific function of the lamp.
The typical lifetime of a deuterium lamp is approximately 2000 hours (Most manufacturers guarantee 2000 hours, but newer lamps are consistently performing well out to 5000 hours and more).
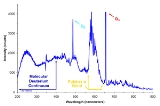
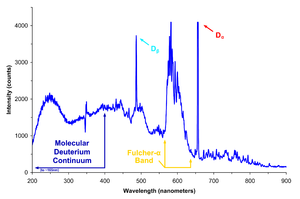
Deuterium lamp spectra
The deuterium lamp emits radiation extending from 112 nm to 900 nm, although its continuous spectrum is only from 180 nm to 370 nm. The spectrum intensity does not actually decrease from 250nm to 300nm as shown in the spectrum plot above. The decrease in the plot is due to decreased efficiency at low wavelengths of the photo detector used to measure the lamp intensity. Deuterium Lamp's continuous spectrum is useful as both a reference in UV radiometric work and to generate a signal in various photometric devices.Safety
Due to the high intensity of UV radiation emitted by the bulb, eye protection is suggested when using a deuterium bulb. Care must also be taken to avoid touching the bulb directly to avoid burns due to high operating temperatureOperating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
s. Touching the bulb directly even when cool can smudge the casing and therefore reduce output intensity.

