Decay product
Encyclopedia
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
, a decay product (also known as a daughter product, daughter isotope or daughter nuclide) is the remaining nuclide
Nuclide
A nuclide is an atomic species characterized by the specific constitution of its nucleus, i.e., by its number of protons Z, its number of neutrons N, and its nuclear energy state....
left over from radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
. Radioactive decay often involves a sequence of steps (decay chain
Decay chain
In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...
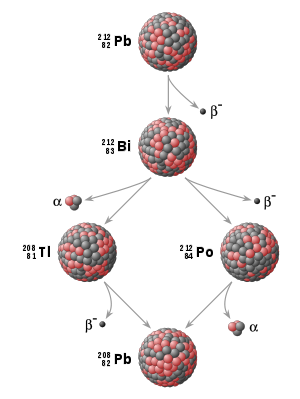
). For example, U-238 decays to Th-234 which decays to Pa-234 which decays, and so on, to Pb-206 (which is stable):

In this example:
- Th-234, Pa-234m,…,Pb-206 are the decay products of U-238.
- Th-234 is the daughter of the parent U-238.
- Pa-234m (234 metastable) is the granddaughter of U-238.
These might also be referred to as the daughter products of U-238.
Decay products are important in understanding radioactive decay and the management of radioactive waste
Radioactive waste
Radioactive wastes are wastes that contain radioactive material. Radioactive wastes are usually by-products of nuclear power generation and other applications of nuclear fission or nuclear technology, such as research and medicine...
.
For elements above lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
in atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
, the decay chain typically ends with an isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of lead.
In many cases members of the decay chain are far more radioactive than the original nuclide. Thus, although uranium is not dangerously radioactive when pure, some pieces of naturally-occurring pitchblende
Uraninite
Uraninite is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2, but also contains UO3 and oxides of lead, thorium, and rare earth elements...
are quite dangerous owing to their radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
content. Similarly, thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
gas mantle
Gas mantle
An incandescent gas mantle, gas mantle, or Welsbach mantle is a device for generating bright white light when heated by a flame. The name refers to its original heat source, existing gas lights, which filled the streets of Europe and North America in the late 19th century, mantle referring to the...
s are very slightly radioactive when new, but become far more radioactive after only a few months of storage.
Although it cannot be predicted whether any given atom of a radioactive substance will decay at any given time, the decay products of a radioactive substance are extremely predictable. Because of this, decay products are important to scientists in many fields who need to know the quantity or type of the parent product. Such studies are done to measure pollution levels (in and around nuclear facilities) and for other matters.

