
De Broglie hypothesis
Encyclopedia
In quantum mechanics
, a matter wave or de Broglie wave (icon) is the wave
(wave–particle duality
) of matter
. The de Broglie relations show that the wavelength
is inversely proportional to the momentum
of a particle and that the frequency
is directly proportional to the particle's kinetic energy
. The wavelength of matter is also called de Broglie wavelength.
The theory was advanced by Louis de Broglie in 1924 in his PhD thesis.
, Albert Einstein
postulated that light was emitted and absorbed as localized packets, or “quanta” (now called photons). These quanta would have an energy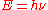 where
where  is the frequency of the light and
is the frequency of the light and 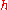 is Planck’s constant. Einstein’s postulate was confirmed experimentally by Robert Millikan
is Planck’s constant. Einstein’s postulate was confirmed experimentally by Robert Millikan
and Arthur Compton
over the next two decades.
Thus it became apparent that light has both wave-like and particle-like properties. De Broglie, in his 1924 PhD thesis, sought to expand this wave-particle duality to all particles:
In 1926, Erwin Schrödinger
published an equation describing how this matter wave should evolve — the matter wave equivalent of Maxwell’s equations — and used it to derive the energy spectrum of hydrogen. That same year Max Born
published his now-standard interpretation that the square of the amplitude of the matter wave gives the probability to find the particle at a given place. This interpretation was in contrast to De Broglie’s own interpretation, in which the wave corresponds to the physical motion of a localized particle.
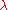 and frequency
and frequency
 to the momentum
to the momentum
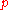 and kinetic energy
and kinetic energy
 , respectively, as
, respectively, as
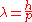 and
and 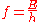
where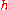 is Planck's constant. The two equations are also written as
is Planck's constant. The two equations are also written as
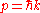

where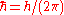 is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),
is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),  is the angular wavenumber defined as
is the angular wavenumber defined as 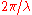 , and
, and  is the angular frequency
is the angular frequency
defined by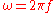 .
.
Using results from special relativity
, the equations can be written as
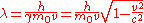
and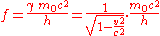
where is the particle's rest mass,
is the particle's rest mass,  is the particle's velocity
is the particle's velocity
, is the Lorentz factor
is the Lorentz factor
, and is the speed of light
is the speed of light
in a vacuum.
See the article on group velocity
for detail on the argument and derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity
(equal to the product of the particle's frequency and its wavelength).
and Lester Germer
fired
slow-moving electrons at a crystalline nickel
target. The angular dependence of the reflected electron intensity was measured, and was determined to have the same diffraction pattern
as those predicted by Bragg
for x-rays. Before the acceptance of the de Broglie hypothesis, diffraction was a property that was thought to be only exhibited by waves. Therefore, the presence of any diffraction
effects by matter demonstrated the wave-like nature of matter. When the de Broglie wavelength was inserted into the Bragg condition
, the observed diffraction pattern was predicted, thereby experimentally confirming the de Broglie hypothesis for electrons.
This was a pivotal result in the development of quantum mechanics
. Just as the photoelectric effect
demonstrated the particle nature of light, the Davisson-Germer experiment
showed the wave-nature of matter, and completed the theory of wave-particle duality. For physicists this idea was important because it means that not only can any particle exhibit wave characteristics, but that one can use wave equation
s to describe phenomena in matter if one uses the de Broglie wavelength.
Since the original Davisson-Germer experiment for electrons, the de Broglie hypothesis has been confirmed for other elementary particles.
The wavelength of a thermalized electron in a non-metal at room temperature is about 8 nm.
and specular reflection
of neutral atoms confirm the application of the de Broglie hypothesis to atoms, i.e. the existence of atomic waves which undergo
diffraction
, interference and allow quantum reflection
by the tails of the attractive potential. Advances in laser cooling
have allowed cooling of neutral atoms down to nanokelvin temperatures. At these temperatures, the thermal de Broglie wavelengths come into the micrometre range. Using Bragg diffraction of atoms and a Ramsey interferometry technique, the de Broglie wavelength of cold sodium
atoms was explicitly measured and found to be consistent with the temperature measured by a different method.
This effect has been used to demonstrate atomic holography
, and it may allow the construction of an atom probe imaging system with nanometer resolution. The description of these phenomena is based on the wave properties of neutral atoms, confirming the de Broglie hypothesis.
demonstrated diffraction for molecules as large as fullerenes. The researchers calculated a De Broglie wavelength of the most probable C60 velocity as 2.5 pm.
More recent experiments prove the quantum nature of molecules with a mass up to 6910 amu.
In general, the De Broglie hypothesis is expected to apply to any well isolated object.
 in a half-space (say,
in a half-space (say,  ), then this observation prevents the particle, which stays in the half-space
), then this observation prevents the particle, which stays in the half-space  from entry into this half-space
from entry into this half-space  . Such an "observation" can be realized with a set of rapidly moving absorbing ridges, filling one half-space. In the system of coordinates related to the ridges, this phenomenon appears as a specular reflection
. Such an "observation" can be realized with a set of rapidly moving absorbing ridges, filling one half-space. In the system of coordinates related to the ridges, this phenomenon appears as a specular reflection
of a particle from a ridged mirror
, assuming the grazing incidence (small values of the grazing angle). Such a ridged mirror is universal; while we consider the idealised "absorption" of the de Broglie wave at the ridges, the reflectivity is determined by wavenumber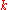 and does not depend on other properties of a particle.
and does not depend on other properties of a particle.
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
, a matter wave or de Broglie wave (icon) is the wave
Wave
In physics, a wave is a disturbance that travels through space and time, accompanied by the transfer of energy.Waves travel and the wave motion transfers energy from one point to another, often with no permanent displacement of the particles of the medium—that is, with little or no associated mass...
(wave–particle duality
Wave–particle duality
Wave–particle duality postulates that all particles exhibit both wave and particle properties. A central concept of quantum mechanics, this duality addresses the inability of classical concepts like "particle" and "wave" to fully describe the behavior of quantum-scale objects...
) of matter
Matter
Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
. The de Broglie relations show that the wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
is inversely proportional to the momentum
Momentum
In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
of a particle and that the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
is directly proportional to the particle's kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
. The wavelength of matter is also called de Broglie wavelength.
The theory was advanced by Louis de Broglie in 1924 in his PhD thesis.
Historical context
At the end of the 19th century, light was thought to consist of waves of electromagnetic fields which propagated according to Maxwell’s equations, while matter was thought to consist of localized particles (See history of wave and particle viewpoints ). This division was challenged when, in his 1905 paper on the photoelectric effectPhotoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
, Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
postulated that light was emitted and absorbed as localized packets, or “quanta” (now called photons). These quanta would have an energy
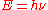 where
where  is the frequency of the light and
is the frequency of the light and 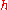 is Planck’s constant. Einstein’s postulate was confirmed experimentally by Robert Millikan
is Planck’s constant. Einstein’s postulate was confirmed experimentally by Robert MillikanRobert Millikan
Robert A. Millikan was an American experimental physicist, and Nobel laureate in physics for his measurement of the charge on the electron and for his work on the photoelectric effect. He served as president of Caltech from 1921 to 1945...
and Arthur Compton
Arthur Compton
Arthur Holly Compton was an American physicist and Nobel laureate in physics for his discovery of the Compton effect. He served as Chancellor of Washington University in St. Louis from 1945 to 1953.-Early years:...
over the next two decades.
Thus it became apparent that light has both wave-like and particle-like properties. De Broglie, in his 1924 PhD thesis, sought to expand this wave-particle duality to all particles:
In 1926, Erwin Schrödinger
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
published an equation describing how this matter wave should evolve — the matter wave equivalent of Maxwell’s equations — and used it to derive the energy spectrum of hydrogen. That same year Max Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
published his now-standard interpretation that the square of the amplitude of the matter wave gives the probability to find the particle at a given place. This interpretation was in contrast to De Broglie’s own interpretation, in which the wave corresponds to the physical motion of a localized particle.
The de Broglie relations
The de Broglie equations relate the wavelength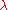 and frequency
and frequencyFrequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
 to the momentum
to the momentumMomentum
In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
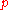 and kinetic energy
and kinetic energyKinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
 , respectively, as
, respectively, as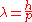 and
and 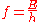
where
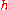 is Planck's constant. The two equations are also written as
is Planck's constant. The two equations are also written as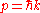

where
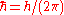 is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),
is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),  is the angular wavenumber defined as
is the angular wavenumber defined as 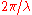 , and
, and  is the angular frequency
is the angular frequencyAngular frequency
In physics, angular frequency ω is a scalar measure of rotation rate. Angular frequency is the magnitude of the vector quantity angular velocity...
defined by
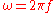 .
.Using results from special relativity
Special relativity
Special relativity is the physical theory of measurement in an inertial frame of reference proposed in 1905 by Albert Einstein in the paper "On the Electrodynamics of Moving Bodies".It generalizes Galileo's...
, the equations can be written as
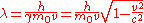
and
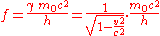
where
 is the particle's rest mass,
is the particle's rest mass,  is the particle's velocity
is the particle's velocityVelocity
In physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
,
 is the Lorentz factor
is the Lorentz factorLorentz factor
The Lorentz factor or Lorentz term appears in several equations in special relativity, including time dilation, length contraction, and the relativistic mass formula. Because of its ubiquity, physicists generally represent it with the shorthand symbol γ . It gets its name from its earlier...
, and
 is the speed of light
is the speed of lightSpeed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
in a vacuum.
See the article on group velocity
Group velocity
The group velocity of a wave is the velocity with which the overall shape of the wave's amplitudes — known as the modulation or envelope of the wave — propagates through space....
for detail on the argument and derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity
Phase velocity
The phase velocity of a wave is the rate at which the phase of the wave propagates in space. This is the speed at which the phase of any one frequency component of the wave travels. For such a component, any given phase of the wave will appear to travel at the phase velocity...
(equal to the product of the particle's frequency and its wavelength).
Elementary particles
In 1927 at Bell Labs, Clinton DavissonClinton Davisson
Clinton Joseph Davisson , was an American physicist who won the 1937 Nobel Prize in Physics for his discovery of electron diffraction. Davisson shared the Nobel Prize with George Paget Thomson, who independently discovered electron diffraction at about the same time as Davisson.-Early...
and Lester Germer
Lester Germer
Lester Halbert Germer was an American physicist. With Clinton Davisson, he proved the wave-particle duality of matter in the Davisson–Germer experiment, which was important to the development of the electron microscope. These studies supported the theoretical work of De Broglie. He also studied...
fired
Davisson-Germer experiment
The Davisson–Germer experiment was a physics experiment conducted by American physicists Clinton Davisson and Lester Germer in 1927, which confirmed the de Broglie hypothesis. This hypothesis advanced by Louis de Broglie in 1924 says that particles of matter such as electrons have wave like...
slow-moving electrons at a crystalline nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
target. The angular dependence of the reflected electron intensity was measured, and was determined to have the same diffraction pattern
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
as those predicted by Bragg
William Lawrence Bragg
Sir William Lawrence Bragg CH OBE MC FRS was an Australian-born British physicist and X-ray crystallographer, discoverer of the Bragg law of X-ray diffraction, which is basic for the determination of crystal structure. He was joint winner of the Nobel Prize for Physics in 1915. He was knighted...
for x-rays. Before the acceptance of the de Broglie hypothesis, diffraction was a property that was thought to be only exhibited by waves. Therefore, the presence of any diffraction
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
effects by matter demonstrated the wave-like nature of matter. When the de Broglie wavelength was inserted into the Bragg condition
Bragg's law
In physics, Bragg's law gives the angles for coherent and incoherent scattering from a crystal lattice. When X-rays are incident on an atom, they make the electronic cloud move as does any electromagnetic wave...
, the observed diffraction pattern was predicted, thereby experimentally confirming the de Broglie hypothesis for electrons.
This was a pivotal result in the development of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
. Just as the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
demonstrated the particle nature of light, the Davisson-Germer experiment
Davisson-Germer experiment
The Davisson–Germer experiment was a physics experiment conducted by American physicists Clinton Davisson and Lester Germer in 1927, which confirmed the de Broglie hypothesis. This hypothesis advanced by Louis de Broglie in 1924 says that particles of matter such as electrons have wave like...
showed the wave-nature of matter, and completed the theory of wave-particle duality. For physicists this idea was important because it means that not only can any particle exhibit wave characteristics, but that one can use wave equation
Wave equation
The wave equation is an important second-order linear partial differential equation for the description of waves – as they occur in physics – such as sound waves, light waves and water waves. It arises in fields like acoustics, electromagnetics, and fluid dynamics...
s to describe phenomena in matter if one uses the de Broglie wavelength.
Since the original Davisson-Germer experiment for electrons, the de Broglie hypothesis has been confirmed for other elementary particles.
The wavelength of a thermalized electron in a non-metal at room temperature is about 8 nm.
Neutral atoms
Experiments with Fresnel diffractionFresnel diffraction
In optics, the Fresnel diffraction equation for near-field diffraction, is an approximation of Kirchhoff-Fresnel diffraction that can be applied to the propagation of waves in the near field....
and specular reflection
Specular reflection
Specular reflection is the mirror-like reflection of light from a surface, in which light from a single incoming direction is reflected into a single outgoing direction...
of neutral atoms confirm the application of the de Broglie hypothesis to atoms, i.e. the existence of atomic waves which undergo
diffraction
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
, interference and allow quantum reflection
Quantum reflection
Quantum reflection is a physical phenomenon involving the reflection of a matter wave from an attractive potential. In classical physics, such a phenomenon is not possible; for instance when one magnet is pulled toward another, you do not expect one of the magnets to suddenly Quantum reflection is...
by the tails of the attractive potential. Advances in laser cooling
Laser cooling
Laser cooling refers to the number of techniques in which atomic and molecular samples are cooled through the interaction with one or more laser light fields...
have allowed cooling of neutral atoms down to nanokelvin temperatures. At these temperatures, the thermal de Broglie wavelengths come into the micrometre range. Using Bragg diffraction of atoms and a Ramsey interferometry technique, the de Broglie wavelength of cold sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
atoms was explicitly measured and found to be consistent with the temperature measured by a different method.
This effect has been used to demonstrate atomic holography
Holography
Holography is a technique that allows the light scattered from an object to be recorded and later reconstructed so that when an imaging system is placed in the reconstructed beam, an image of the object will be seen even when the object is no longer present...
, and it may allow the construction of an atom probe imaging system with nanometer resolution. The description of these phenomena is based on the wave properties of neutral atoms, confirming the de Broglie hypothesis.
Waves of molecules
Recent experiments even confirm the relations for molecules and even macromolecules, which are normally considered too large to undergo quantum mechanical effects. In 1999, a research team in ViennaVienna
Vienna is the capital and largest city of the Republic of Austria and one of the nine states of Austria. Vienna is Austria's primary city, with a population of about 1.723 million , and is by far the largest city in Austria, as well as its cultural, economic, and political centre...
demonstrated diffraction for molecules as large as fullerenes. The researchers calculated a De Broglie wavelength of the most probable C60 velocity as 2.5 pm.
More recent experiments prove the quantum nature of molecules with a mass up to 6910 amu.
In general, the De Broglie hypothesis is expected to apply to any well isolated object.
Spatial Zeno effect
The matter wave leads to the spatial version of the Zeno effect. If an object (particle) is observed with frequency in a half-space (say,
in a half-space (say,  ), then this observation prevents the particle, which stays in the half-space
), then this observation prevents the particle, which stays in the half-space  from entry into this half-space
from entry into this half-space  . Such an "observation" can be realized with a set of rapidly moving absorbing ridges, filling one half-space. In the system of coordinates related to the ridges, this phenomenon appears as a specular reflection
. Such an "observation" can be realized with a set of rapidly moving absorbing ridges, filling one half-space. In the system of coordinates related to the ridges, this phenomenon appears as a specular reflectionSpecular reflection
Specular reflection is the mirror-like reflection of light from a surface, in which light from a single incoming direction is reflected into a single outgoing direction...
of a particle from a ridged mirror
Ridged mirror
In atomic physics, a ridged mirror is a kind of atomic mirror, designed for the specular reflection of neutral particles coming at the grazing incidence angle, characterised in the following: in order to reduce the mean attraction of particles to the surface and increase the reflectivity, this...
, assuming the grazing incidence (small values of the grazing angle). Such a ridged mirror is universal; while we consider the idealised "absorption" of the de Broglie wave at the ridges, the reflectivity is determined by wavenumber
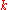 and does not depend on other properties of a particle.
and does not depend on other properties of a particle.See also
- Atom opticsAtom opticsAtom optics is the area of physics which deals with beams of cold, slowly moving neutral atoms, as a special case of a particle beam....
- Atomic de Broglie microscope
- Atomic mirrorAtomic mirror (physics)In physics, an atomic mirror is a device which reflects neutral atoms in the similar way as the conventional mirror reflects visible light. Atomic mirrors can be made of electric fields or magnetic fields, electromagnetic waves or just silicon wafer; in the last case, atoms are reflected by the...
- Bohr modelBohr modelIn atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
- Faraday waveFaraday waveFaraday waves, also known as Faraday ripples, named after Michael Faraday, are nonlinear standing waves that appear on liquids enclosed by a vibrating receptacle. When the vibration frequency exceeds a critical value, the flat hydrostatic surface becomes unstable. This is known as the Faraday...
- Quantum reflectionQuantum reflectionQuantum reflection is a physical phenomenon involving the reflection of a matter wave from an attractive potential. In classical physics, such a phenomenon is not possible; for instance when one magnet is pulled toward another, you do not expect one of the magnets to suddenly Quantum reflection is...
- Ridged mirrorRidged mirrorIn atomic physics, a ridged mirror is a kind of atomic mirror, designed for the specular reflection of neutral particles coming at the grazing incidence angle, characterised in the following: in order to reduce the mean attraction of particles to the surface and increase the reflectivity, this...
- Theoretical and experimental justification for the Schrödinger equationTheoretical and experimental justification for the Schrödinger equationThe theoretical and experimental justification for the Schrödinger equation motivates the discovery of the Schrödinger equation, the equation that describes the dynamics of nonrelativistic particles...
- Thermal de Broglie wavelength
- Zeno effect
Further reading
- Broglie, Louis de, The wave nature of the electron Nobel Lecture, 12, 1929
- Tipler, Paul A. and Ralph A. Llewellyn (2003). Modern Physics. 4th ed. New York; W. H. Freeman and Co. ISBN 0-7167-4345-0. pp. 203–4, 222–3, 236.
- Web version of Thesis, translated (English)
- Steven S. Zumdahl, Chemical Principles 5th Edition, (2005) Houghton Mifflin Company.
- An extensive review article "Optics and interferometry with atoms and molecules" appeared in July 2009: http://www.atomwave.org/rmparticle/RMPLAO.pdf.

