
DART ion source
Encyclopedia

Atmospheric pressure
Atmospheric pressure is the force per unit area exerted into a surface by the weight of air above that surface in the atmosphere of Earth . In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point...
ion source
Ion source
An ion source is an electro-magnetic device that is used to create charged particles. These are used primarily to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.- Electron ionization :...
that instantaneously ionizes gases, liquids and solids in open air under ambient
Ambient pressure
The ambient pressure on an object is the pressure of the surrounding medium, such as a gas or liquid, which comes into contact with the object....
conditions. It was developed in 2005 by Laramee and Cody and is now marketed commercially by JEOL
JEOL
is a manufacturer of electron microscopes and other scientific instruments. Its headquarters are in Tokyo, Japan, with 25 subsidiaries and two associated companies....
and IonSense
Ionsense
IonSense, Inc. is a Massachusetts based company that is developing technology for the analysis of materials by direct analysis in real time or DART mass spectrometry...
. It was among the first ambient ionization
Ambient ionization
Ambient ionization is a form of ionization in which ions are formed outside the mass spectrometer without sample preparation or separation.-Direct analysis in real time:...
techniques not requiring sample preparation, so solid and liquid materials can be analyzed by mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
in their native state. Ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
can take place directly on the sample surface, such as, currency bills, tablets, bodily fluids (blood, saliva and urine), glass, plant leaves, fruits & vegetables and even clothing. Liquids are analyzed by dipping an object (such as a glass rod) into the liquid sample and then presenting it to the DART ion source. Vapors are introduced directly into the DART gas stream.
Ionization process
The ionization process involves an interaction between the analyteAnalyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
(S) and electronically excited atoms or vibronically excited molecules (metastable species – M*):

Upon collision
Collision
A collision is an isolated event which two or more moving bodies exert forces on each other for a relatively short time.Although the most common colloquial use of the word "collision" refers to accidents in which two or more objects collide, the scientific use of the word "collision" implies...
between the excited gas molecule (M*) and the surface of the sample, an energy transfer takes place, from the excited gas molecule (M*) to the neutral analyte molecule (S). This causes an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
to be released from the analyte molecule, producing a radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
cation
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
. The molecular cation is then ejected from the sampling surface and travels to the mass analyzer along with the gas stream (typically N2
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or Ne
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
). The process presented in the above equation is called Penning ionization
Penning ionization
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms and/or molecules. The process is named after the Dutch physicist Frans Michel Penning who first reported it in 1927....
. For this ionization process to take place, the energy of the excited state gas molecule must be higher than the ionization potential
Ionization potential
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
of the neutral molecule.
When He
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
is used as the carrier gas, the ionization process occurs by the following mechanism:
First an excited state He atom collides with an atmospheric pressure water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
molecule and ionizes it:

The ionized water molecule then undergoes several reactions with other neutral water molecules resulting in the formation of a protonated
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
water cluster
Cluster
-In science:* Cluster , a small group of atoms or molecules* Cluster chemistry, an array of bound atoms intermediate in character between a molecule and a solid...
:


The water cluster then interacts with the analyte molecule (S) generating a protonated molecule.
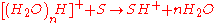
DART can also operate in the negative ion mode by which negatively charged species are formed. The negative ion formation process is under current discussion and investigation.

Formation of metastable species
As the gas (N2, Ne or He) enters the ion source, an electric potentialElectric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
in the range of +1 to +5 kV is applied. This generates a glow discharge containing ionized gas, electrons and excited state atoms/molecules (metastable species). A potential of 100 V applied to the electrostatic
Electrostatics
Electrostatics is the branch of physics that deals with the phenomena and properties of stationary or slow-moving electric charges....
lenses removes charged particles from the gas stream and only excited state species flow to the third chamber. The gas stream in the third chamber can be heated from RT to 250 oC. Heating is optional but may be necessary depending on the surface or chemical being analyzed. An insulator cap at the terminal end of the ion source protects the operator from harm.
The excited-state species can interact directly with the sample which can be a solid, liquid or gas to desorb and ionize the analyte.
The distance between the ion source and the inlet of the mass spectrometer is 5 to 25 mm.
The ions formed are directed to the mass spectrometer inlet by both the gas flow and a slight vacuum in the spectrometer inlet. Although optimum geometries exist for specific applications, the exact positioning, distance and angle of DART ion source with respect to the sample surface and the mass spectrometer inlet are not critical.
Source to analyzer interface
Ions entering the mass spectrometer first go through a source - to - analyzer interface, which was designed in order to minimize spectrometer contamination.The ions are directed to the ion guide through orifice 1 and 2 by applying a slight potential difference between them: orifice 1 - 30V and orifice 2 - 5V.
It is clear from the diagram that the space between the two orifices is not horizontal but rather diagonal. Species containing charge (ions) are attracted to the second orifice, but neutral molecules travel in a straight pathway and thus get trapped in that region. The contamination is then removed by the pump.
Mass spectra
DART produces relatively simple mass spectra, dominated by protonated molecules [M+H]+ in positive-ion mode, or deprotonated molecules [M-H]- in negative-ion mode. Depending on the nature of the molecule, other species may be formed, such as M+. from polynuclear aromatic hydrocarbons. Fragmentation may occasionally be observed for some molecules. Multiple-charge ions and alkali metal cation aducts are never observed, but addition of ammonia or other "dopants" to the DART gas stream can be used to form single-charge adducts such as [M+NH4]+ or [M+Cl]- for compounds that would not readily form molecular ions or protonated molecules. For example, the explosives nitroglycerin and HMX do not form [M-H]-,but readily form [M+Cl]- if chloride is present.Applications
DART can be applied to a wide range of applications, such as, the fragrance industry, pharmaceutical industry, foods and spices, forensic science and health.In the fragrance industry, the deposition and release of a fragrance on surfaces such as, fabric and hair is often studied. Use of DART compared to traditional methods minimizes sample amount, sample preparation, eliminates extraction steps, decreases limit of detection and analysis time.
In the pharmaceutical industry, there is a growing international problem, which is the production of counterfeit drugs. Some countries in which this occurs are United Kingdom, China, Russia, Argentina, Nigeria and India. Dart can detect active ingredients in medicine in a tablet form; there is no need for sample preparation such as, crushing or extracting.
DART was used to directly analyze a red pepper pod in three different places: the membrane (white part holding the seeds), the seeds and the flesh of the pepper. The analyte of interest was capsaicin, a natural ingredient of a red pepper pod that is responsible for the burning sensation when eating chilies. The spectrum obtained revealed that the highest concentration of capsaicin is in the membrane. DART has recently been used in the study of genus Allium plants, e.g., to identify the lachrymatory compound, syn-propanethial S-oxide, C3H6OS, in onion, Allium cepa, a previously unknown lachrymatory compound, syn-butanethial S-oxide, C4H8OS, in Allium siculum, and 2-propenesulfenic acid, an isomer of propanethial S-oxide, which is the very short-lived precursor to allicin from cutting garlic, Allium sativum.
See also
- Desorption electrospray ionizationDesorption electrospray ionizationDesorption electrospray ionization is an ambient ionization technique that can be used in mass spectrometry for chemical analysis. It is an atmospheric pressure ion source that ionizes gases, liquids and solids in open air under ambient conditions. It was developed in 2004 by Professor Graham...
- Electric glow dischargeElectric glow dischargeAn electric glow discharge is a plasma formed by the passage of current at 100 V to several kV through a gas, often argon or another noble gas. It is found in products such as neon lamps and plasma-screen televisions, and is used in plasma physics and analytical chemistry.-Basic operating...
- Atmospheric pressure chemical ionizationAtmospheric pressure chemical ionizationAtmospheric-pressure chemical ionization is an ionization method used in mass spectrometry. It is a form of chemical ionization which takes place at atmospheric pressure.-How it works:...
- Desorption atmospheric pressure photoionizationDesorption atmospheric pressure photoionizationDesorption atmospheric pressure photoionization is an atmospheric-pressure ionization technique for mass spectrometry. DAPPI enables direct analysis of solid samples without pretreatment and analysis of samples deposited on surfaces by means of a jet of hot solvent vapour and vacuum ultraviolet...
Patents
- Robert B. Cody and James A. Laramee, “Method for atmospheric pressure ionization” issued September 27, 2005. (Priority date: April 2003).
- James A. Laramee and Robert B. Cody “Method for Atmospheric Pressure Analyte Ionization” issued September 26, 2006.

