
Cytochrome c oxidase
Encyclopedia
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
cytochrome c oxidase or Complex IV is a large transmembrane protein
Transmembrane protein
A transmembrane protein is a protein that goes from one side of a membrane through to the other side of the membrane. Many TPs function as gateways or "loading docks" to deny or permit the transport of specific substances across the biological membrane, to get into the cell, or out of the cell as...
complex found in bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and the mitochondrion
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
.
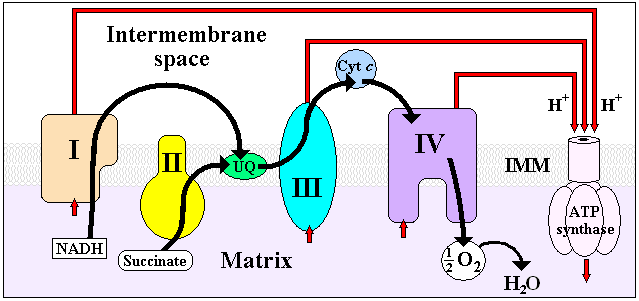
It is the last enzyme in the respiratory electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
of mitochondria (or bacteria) located in the mitochondrial (or bacterial) membrane. It receives an electron from each of four cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
molecules, and transfers them to one oxygen molecule, converting molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to two molecules of water. In the process, it binds four protons from the inner aqueous phase to make water, and in addition translocates four protons across the membrane, helping to establish a transmembrane difference of proton electrochemical potential that the ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
then uses to synthesize ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
.
Structure

Integral membrane protein
An integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
composed of several metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
prosthetic sites and 13 protein subunits in mammals. In mammals, ten subunits are nuclear in origin, and three are synthesized in the mitochondria. The complex contains two heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
s, a cytochrome a and cytochrome a3, and two copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
centers, the CuA and CuB centers. In fact, the cytochrome a3 and CuB form a binuclear center that is the site of oxygen reduction. Cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
reduced by the preceding component of the respiratory chain (cytochrome bc1 complex, complex III) docks near the CuA binuclear center, passing an electron to it and being oxidized back to cytochrome c containing Fe3+. The reduced CuA binuclear center now passes an electron on to cytochrome a, which in turn passes an electron on to the cytochrome a3- CuB binuclear center. The two metal ions in this binuclear center are 4.5 Å apart and coordinate a hydroxide ion in the fully oxidized state.
Crystallographic studies
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
of cytochrome c oxidase show an unusual post-translational modification, linking C6 of Tyr(244) and the ε-N of His(240) (bovine enzyme numbering). It plays a vital role in enabling the cytochrome a3- CuB binuclear center to accept four electrons in reducing molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. The mechanism of reduction was formerly thought to involve a peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
intermediate, which was believed to lead to superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
production. However, the currently accepted mechanism involves a rapid four-electron reduction involving immediate oxygen-oxygen bond cleavage, avoiding any intermediate likely to form superoxide.
Assembly
Site of assembly is believed to occur near TOMMitochondrial membrane transport protein
Mitochondrial membrane transport proteins are proteins which exist in the membranes of mitochondria and which serve to transport molecules and other factors such as ions into or out of the organelles.-Examples:...
/TIM, where complex intermediates are accessible to bind with subunits imported from cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
. Hemes and cofactors are inserted into subunits I & II Subunits I and IV initiate assembly. Other subunits may form sub-complex intermediates that later bind to others to form COX complex. In post-assembly modifications, the enzyme is dimerized, which is required for active/efficient enzyme action. Dimers are connected by a cardiolipin
Cardiolipin
Cardiolipin is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. The only other place that cardiolipin can be found is in the membranes of most bacteria. The name ‘cardiolipin’ is derived from the fact that it was first...
molecule.
Biochemistry
Summary reaction:- 4 Fe2+-cytochrome c + 8 H+in + O2 → 4 Fe3+-cytochrome c + 2 H2O + 4 H+out
Two electrons are passed from two cytochrome c's, through the CuA and cytochrome a sites to the cytochrome a3- CuB binuclear center, reducing the metals to the Fe+2 form and Cu+1. The hydroxide ligand is protonated and lost as water, creating a void between the metals that is filled by O2. The oxygen is rapidly reduced, with two electrons coming from the Fe+2cytochrome a3, which is converted to the ferryl oxo form (Fe+4=O). The oxygen atom close to CuB picks up one electron from Cu+1, and a second electron and a proton from the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
of Tyr(244), which becomes a tyrosyl radical: The second oxygen is converted to a hydroxide ion by picking up two electrons and a proton. A third electron arising from another cytochrome c is passed through the first two electron carriers to the cytochrome a3- CuB binuclear center, and this electron and two protons convert the tyrosyl radical back to Tyr, and the hydroxide bound to CuB+2 to a water molecule. The fourth electron from another cytochrome c flows through CuA and cytochrome a to the cytochrome a3- CuB binuclear center, reducing the Fe+4=O to Fe+3, with the oxygen atom picking up a proton simultaneously, regenerating this oxygen as a hydroxide ion coordinated in the middle of the cytochrome a3- CuB center as it was at the start of this cycle. The net process is that four reduced cytochrome c's are used, along with 4 protons, to reduce O2 to two water molecules.
Inhibition
CyanideCyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
, sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
, azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
, and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
all bind to cytochrome c oxidase, thus competitively
Competitive inhibition
Competitive inhibition is a form of enzyme inhibition where binding of the inhibitor to the active site on the enzyme prevents binding of the substrate and vice versa.-Mechanism:...
inhibiting the protein from functioning, which results in chemical asphyxiation of cells. Methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
in methylated spirits is converted into formic acid, which also inhibits the same oxidase system.
Genetic defects and disorders
Defects involving genetic mutations altering cytochrome c oxidase (COX) functionality or structure can result in severe, often fatal metabolic disorders. Such disorders usually manifest in early childhood and affect predominantly tissues with high energy demands (brain, heart, muscle). Among the many classified mitochondrial diseases, those involving dysfunctional COX assembly are thought to be the most severe.The vast majority of COX disorders are linked to mutations in nuclear-encoded proteins referred to as assembly factors, or assembly proteins. These assembly factors contribute to COX structure and functionality, and are involved in several essential processes, including transcription and translation of mitochondrion-encoded subunits, processing of preproteins and membrane insertion, and cofactor biosynthesis and incorporation.
Currently, mutations have been identified in six COX assembly factors: SURF1
SURF1
Surfeit locus protein 1 is a protein that in humans is encoded by the SURF1 gene.-Further reading:...
, SCO1
SCO1
Protein SCO1 homolog, mitochondrial is a protein that in humans is encoded by the SCO1 gene.Mutations in both SCO1 and SCO2 are associated with distinct clinical phenotypes as well as tissue-specific cytochrome c oxidase deficiency. SCO1 localizes predominantly to blood vessels, whereas SCO2 is...
, SCO2
SCO2
Protein SCO2 homolog, mitochondrial is a protein that in humans is encoded by the SCO2 gene.-Further reading:...
, COX10
COX10
Protoheme IX farnesyltransferase, mitochondrial is an enzyme that in humans is encoded by the COX10 gene.-Further reading:...
, COX15
COX15
Cytochrome c oxidase assembly protein COX15 homolog is an enzyme that in humans is encoded by the COX15 gene.-Further reading:...
, and LRPPRC
LRPPRC
Leucine-rich PPR motif-containing protein, mitochondrial is a protein that in humans is encoded by the LRPPRC gene. Transcripts ranging in size from 4.8 to 7.0 kb which result from alternative polyadenylation have been reported for this gene.-Function:...
. Mutations in these proteins can result in altered functionality of sub-complex assembly, copper transport, or translational regulation. Each gene mutation is associated with the etiology of a specific disease, with some having implications in multiple disorders. Disorders involving dysfunctional COX assembly via gene mutations include Leigh syndrome, cardiomyopathy
Cardiomyopathy
Cardiomyopathy, which literally means "heart muscle disease," is the deterioration of the function of the myocardium for any reason. People with cardiomyopathy are often at risk of arrhythmia or sudden cardiac death or both. Cardiomyopathy can often go undetected, making it especially dangerous to...
, leukodystrophy
Leukodystrophy
Leukodystrophy refers to a group of disorders characterized by dysfunction of the white matter of the brain. The leukodystrophies are caused by imperfect growth or development of the myelin sheath, the fatty covering that acts as an insulator around nerve fibers...
, anemia
Anemia
Anemia is a decrease in number of red blood cells or less than the normal quantity of hemoglobin in the blood. However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin...
, and sensorineural deafness.
Histochemistry
COX histochemistry is used for mapping regional brain metabolism in animals, since there is a direct relation between enzyme activity and neuronal activity. Such brain mapping has been accomplished in spontaneous mutant mice with cerebellar disease such as reelerReeler
A reeler is a mouse mutant, so named because of its characteristic "reeling" gait. This is caused by profound hypoplasia of the mouse's cerebellum, in which the normal cerebellar folia are missing. The mutation is autosomal and recessive....
and a transgenic model of Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
. This technique has also been used to map learning activity in animal brain.
See also
- Cytochrome c oxidase subunit I
- Cytochrome c oxidase subunit IICytochrome c oxidase subunit IICytochrome c oxidase subunit II, abbreviated CoxII, is the second subunit of cytochrome c oxidase.Cytochrome c oxidase is an oligomeric enzymatic complex which is a component of the respiratory chain and is involved in the transfer of electrons from cytochrome c to oxygen...
- Cytochrome c oxidase subunit IIICytochrome c oxidase subunit IIICytochrome c oxidase subunit III is one of main transmembrane subunits of cytochrome c oxidaseCytochrome c oxidase is the terminal enzyme of the respiratory chain of mitochondria and many aerobic bacteria...
- Heme aHeme aHeme A is a heme, a coordination complex consisting of a macrocyclic ligand called a porphyrin, chelating an iron atom. Heme a is a biomolecule and is produced naturally by many organisms.-Relationship to other hemes:...
External links
- The Cytochrome Oxidase home page at Rice UniversityRice UniversityWilliam Marsh Rice University, commonly referred to as Rice University or Rice, is a private research university located on a heavily wooded campus in Houston, Texas, United States...
- Interactive Molecular model of cytochrome c oxidase (Requires MDL Chime)

