
Cyanogen bromide
Encyclopedia
Cyanogen bromide is a pseudohalogen
compound
with the formula
CNBr. It is a colorless solid that is widely used to modify biopolymer
s, fragment protein
s and peptide
s, and synthesize other compounds.
atom is actually bonded to bromine
by a single bond and to nitrogen
by a triple bond
(i.e. Br–C≡N). The compound is linear and quite polar, but it does not spontaneously ionize in water. Therefore, it dissolves in both water and polar organic solvents.
Cyanogen bromide can be prepared by oxidation of sodium cyanide
with bromine
, which proceeds in two steps via the intermediate cyanogen
((CN)2 or N≡C–C≡N).
2 + Br2 → 2 BrCN
Cyanogen bromide is hydrolyzed by water to release hydrogen cyanide and hypobromous acid
s, and synthesize cyanamide
s and other molecules.
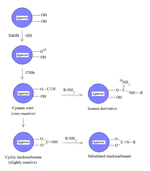
s such as agarose for affinity chromatography
. Because of its simplicity and mild pH
conditions, cyanogen bromide activation is the most common method for preparing affinity gels. CNBr is also often used because it reacts with the hydroxyl
groups on agarose to form cyanate
ester
s and imidocarbonates. These groups are reacted with primary amines in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding isourea derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a ligand
to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak anion exchangers.
s at the C-terminus of methionine
residues. This reaction is used to reduce the size of polypeptide segments for identification and sequencing
.
is shifted away from the carbon atom, making it unusually electrophilic, and towards the more electronegative bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a nucleophile
, and the cleavage reaction begins with a nucleophilic acyl substitution
reaction in which bromine is ultimately replaced by the sulfur in methionine. This attack is followed by the formation of a five-membered ring as opposed to a six-membered ring, which would entail the formation of a double bond
in the ring between nitrogen and carbon. This double bond would result in a rigid ring conformation, thereby destabilizing the molecule. Thus, the five-membered ring is formed so that the double bond is outside the ring, as shown in the figure.
Although the nucleophilic sulfur in methionine is responsible for attacking CNBr, the sulfur in cysteine
does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide adduct
, leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide nitrogen, which, if attacked by water, would yield cyanic acid and the original cysteine.
such as 0.1M HCl (hydrochloric acid
) or 70% (formic acid
). These are the most common buffers for cleavage. An advantage to HCl is that formic acid causes the formation of formyl esters, which complicates protein characterization. However, formic is still often used because it dissolves most proteins. Also, the oxidation of methionine to methionine sulfoxide, which is inert to CNBr attack, occurs more readily in HCl than in formic acid, possibly because formic acid is a reducing acid. Alternative buffers for cleavage include guanidine
or urea
in HCl because of their ability to unfold proteins
, thereby making methionine more accessible to CNBr.
Note that water is required for normal peptide bond cleavage of the iminolactone intermediate. In formic acid, cleavage of Met-Ser
and Met-Thr
bonds is enhanced with increased water concentration because these conditions favor the addition of water across the imine
rather than reaction of the side chain hydroxyl with the imine. Lowered pH tends to increase cleavage rates by inhibiting methionine side chain oxidation.
or threonine
, side reactions can occur that destroy the methionine without peptide bond cleavage
. Normally, once the iminolactone is formed (refer to figure), water and acid can react with the imine to cleave the peptide bond, forming a homoserine lactone
and new C-terminal peptide. However, if the adjacent amino acid to methionine has a hydroxyl
or sulfhydryl group, this group can react with the imine to form a homoserine without peptide bond cleavage. These two cases are shown in the figure.
. As stated earlier, the reagent is prone to attack by nucleophiles such as amines and alcohols because of the electrophilic carbon. In the synthesis of cyanamide
s and dicyanamides, primary and secondary amines react with CNBr to yield mono- and dialkylcyanamides, which can further react with amines and hydroxylamine
to yield guanidine
s and hydroxyguanidines. In the von Braun reaction
, tertiary amines react with CNBr to yield disubstituted cyanamides and an alkyl bromide. CNBr can be used to prepare aryl
nitrile
s, nitriles, anhydrides, and cyanate
s. It can also serve as a cleaving agent.
Cyanogen bromide is volatile, and readily absorbed through the skin
or gastrointestinal tract
. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of nonspecific symptoms. Exposure to even small amounts may cause convulsions or death. LD50 orally in rats is reported as 25–50 mg/kg.
To deactivate CNBr in a solution not exceeding 60 g/L of CNBr (dilute if necessary), the recommended method is to add 1 mol/L NaOH and 1 mol/L NaOCl in volumes of ratio 1:1:2 (CNBr solution:NaOH:NaOCl). The aqueous alkali hydroxide instantly hydrolyzes CNBr to alkali cyanide and bromide. The cyanide can then be oxidized by sodium or calcium hypochlorite
to the less toxic cyanate ion. Note that deactivation is extremely exothermic
and may be explosive.
Pseudohalogen
Pseudo'halogen molecules are inorganic molecules of the general formsPs–Ps or Ps–X, where Ps is a pseudohalogen group such as cyanide, cyanate, thiocyanate and others, and X is a "true" halogen...
compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
CNBr. It is a colorless solid that is widely used to modify biopolymer
Biopolymer
Biopolymers are polymers produced by living organisms. Since they are polymers, Biopolymers contain monomeric units that are covalently bonded to form larger structures. There are three main classes of biopolymers based on the differing monomeric units used and the structure of the biopolymer formed...
s, fragment protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s and peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s, and synthesize other compounds.
Synthesis, basic properties, and structure
Although the formula is most commonly written CNBr, the carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom is actually bonded to bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
by a single bond and to nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
by a triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
(i.e. Br–C≡N). The compound is linear and quite polar, but it does not spontaneously ionize in water. Therefore, it dissolves in both water and polar organic solvents.
Cyanogen bromide can be prepared by oxidation of sodium cyanide
Sodium cyanide
Sodium cyanide is an inorganic compound with the formula NaCN. This highly toxic colorless salt is used mainly in gold mining but has other niche applications...
with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, which proceeds in two steps via the intermediate cyanogen
Cyanogen
Cyanogen is the chemical compound with the formula 2. It is a colorless, toxic gas with a pungent odor.The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups — analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing...
((CN)2 or N≡C–C≡N).
- 2 NaCN + Br2 → (CN)2 + 2 NaBr
2 + Br2 → 2 BrCN
Cyanogen bromide is hydrolyzed by water to release hydrogen cyanide and hypobromous acid
Hypobromous acid
Hypobromous acid is a weak, unstable acid with chemical formula HBrO. It is also called bromic acid, bromanol or hydroxidobromine. It occurs only in solution and has chemical and physical properties that are very similar to those of hypochlorous acid.In aqueous solution, hypobromous acid partially...
- BrCN + H2O → HCN + HOBr
Biochemical applications
The main uses of cyanogen bromide are to immobilize proteins, fragment proteins by cleaving peptide bondPeptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s, and synthesize cyanamide
Cyanamide
Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug in Canada, Europe and Japan. The molecule features a nitrile group attached to an...
s and other molecules.

Protein immobilization
Cyanogen bromide is often used to immobilize proteins by coupling them to reagentReagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s such as agarose for affinity chromatography
Affinity chromatography
Affinity chromatography is a method of separating biochemical mixtures and based on a highly specific interaction such as that between antigen and antibody, enzyme and substrate, or receptor and ligand.-Uses:Affinity chromatography can be used to:...
. Because of its simplicity and mild pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
conditions, cyanogen bromide activation is the most common method for preparing affinity gels. CNBr is also often used because it reacts with the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups on agarose to form cyanate
Cyanate
The cyanate ion is an anion with the chemical formula written as [OCN]− or [NCO]−. In aqueous solution it acts as a base, forming isocyanic acid, HNCO. The cyanate ion is an ambidentate ligand, forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s and imidocarbonates. These groups are reacted with primary amines in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding isourea derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak anion exchangers.
Protein cleavage
Cyanogen bromide hydrolyzes peptide bondPeptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s at the C-terminus of methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
residues. This reaction is used to reduce the size of polypeptide segments for identification and sequencing
Protein sequencing
Protein sequencing is a technique to determine the amino acid sequence of a protein, as well as which conformation the protein adopts and the extent to which it is complexed with any non-peptide molecules...
.
Mechanism
In CNBr, the electron densityElectron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
is shifted away from the carbon atom, making it unusually electrophilic, and towards the more electronegative bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
, and the cleavage reaction begins with a nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
reaction in which bromine is ultimately replaced by the sulfur in methionine. This attack is followed by the formation of a five-membered ring as opposed to a six-membered ring, which would entail the formation of a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
in the ring between nitrogen and carbon. This double bond would result in a rigid ring conformation, thereby destabilizing the molecule. Thus, the five-membered ring is formed so that the double bond is outside the ring, as shown in the figure.
Although the nucleophilic sulfur in methionine is responsible for attacking CNBr, the sulfur in cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
, leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide nitrogen, which, if attacked by water, would yield cyanic acid and the original cysteine.
Reaction conditions
Cleaving proteins with CNBr requires using a bufferBuffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
such as 0.1M HCl (hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
) or 70% (formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
). These are the most common buffers for cleavage. An advantage to HCl is that formic acid causes the formation of formyl esters, which complicates protein characterization. However, formic is still often used because it dissolves most proteins. Also, the oxidation of methionine to methionine sulfoxide, which is inert to CNBr attack, occurs more readily in HCl than in formic acid, possibly because formic acid is a reducing acid. Alternative buffers for cleavage include guanidine
Guanidine
Guanidine is a crystalline compound of strong alkalinity formed by the oxidation of guanine. It is used in the manufacture of plastics and explosives. It is found in urine as a normal product of protein metabolism. The molecule was first synthesized in 1861 by the oxidative degradation of an...
or urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
in HCl because of their ability to unfold proteins
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
, thereby making methionine more accessible to CNBr.
Note that water is required for normal peptide bond cleavage of the iminolactone intermediate. In formic acid, cleavage of Met-Ser
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
and Met-Thr
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
bonds is enhanced with increased water concentration because these conditions favor the addition of water across the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
rather than reaction of the side chain hydroxyl with the imine. Lowered pH tends to increase cleavage rates by inhibiting methionine side chain oxidation.
Side reactions
When methionine is followed by serineSerine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
or threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, side reactions can occur that destroy the methionine without peptide bond cleavage
Bond cleavage
Bond cleavage, or scission, is the splitting of chemical bonds.If the two electrons in a cleaved covalent bond are divided between the products, the process is known as homolytic fission and free redicals are generated by homolytic cleavage the process is known as homolytic fission or homolysis...
. Normally, once the iminolactone is formed (refer to figure), water and acid can react with the imine to cleave the peptide bond, forming a homoserine lactone
Homoserine Lactone
N-Acyl homoserine lactones are a class of signaling molecules involved in bacterial quorum sensing. Quorum sensing is a method of communication between bacteria that enables the coordination of group-based behavior based on population density...
and new C-terminal peptide. However, if the adjacent amino acid to methionine has a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
or sulfhydryl group, this group can react with the imine to form a homoserine without peptide bond cleavage. These two cases are shown in the figure.
Organic synthesis
Cyanogen bromide is also widely used in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. As stated earlier, the reagent is prone to attack by nucleophiles such as amines and alcohols because of the electrophilic carbon. In the synthesis of cyanamide
Cyanamide
Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug in Canada, Europe and Japan. The molecule features a nitrile group attached to an...
s and dicyanamides, primary and secondary amines react with CNBr to yield mono- and dialkylcyanamides, which can further react with amines and hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
to yield guanidine
Guanidine
Guanidine is a crystalline compound of strong alkalinity formed by the oxidation of guanine. It is used in the manufacture of plastics and explosives. It is found in urine as a normal product of protein metabolism. The molecule was first synthesized in 1861 by the oxidative degradation of an...
s and hydroxyguanidines. In the von Braun reaction
Von Braun reaction
The Von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide. An example is the reaction of dimethyl-α-naphthylamine :...
, tertiary amines react with CNBr to yield disubstituted cyanamides and an alkyl bromide. CNBr can be used to prepare aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s, nitriles, anhydrides, and cyanate
Cyanate
The cyanate ion is an anion with the chemical formula written as [OCN]− or [NCO]−. In aqueous solution it acts as a base, forming isocyanic acid, HNCO. The cyanate ion is an ambidentate ligand, forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair...
s. It can also serve as a cleaving agent.
Toxicity, storage, and deactivation
Cyanogen bromide is moisture-sensitive but can be stored under dry conditions at 2 to 8 °C for extended periods of time.Cyanogen bromide is volatile, and readily absorbed through the skin
Skin
-Dermis:The dermis is the layer of skin beneath the epidermis that consists of connective tissue and cushions the body from stress and strain. The dermis is tightly connected to the epidermis by a basement membrane. It also harbors many Mechanoreceptors that provide the sense of touch and heat...
or gastrointestinal tract
Gastrointestinal tract
The human gastrointestinal tract refers to the stomach and intestine, and sometimes to all the structures from the mouth to the anus. ....
. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of nonspecific symptoms. Exposure to even small amounts may cause convulsions or death. LD50 orally in rats is reported as 25–50 mg/kg.
To deactivate CNBr in a solution not exceeding 60 g/L of CNBr (dilute if necessary), the recommended method is to add 1 mol/L NaOH and 1 mol/L NaOCl in volumes of ratio 1:1:2 (CNBr solution:NaOH:NaOCl). The aqueous alkali hydroxide instantly hydrolyzes CNBr to alkali cyanide and bromide. The cyanide can then be oxidized by sodium or calcium hypochlorite
Calcium hypochlorite
Calcium hypochlorite is a chemical compound with formula 2. It is widely used for water treatment and as a bleaching agent...
to the less toxic cyanate ion. Note that deactivation is extremely exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
and may be explosive.

